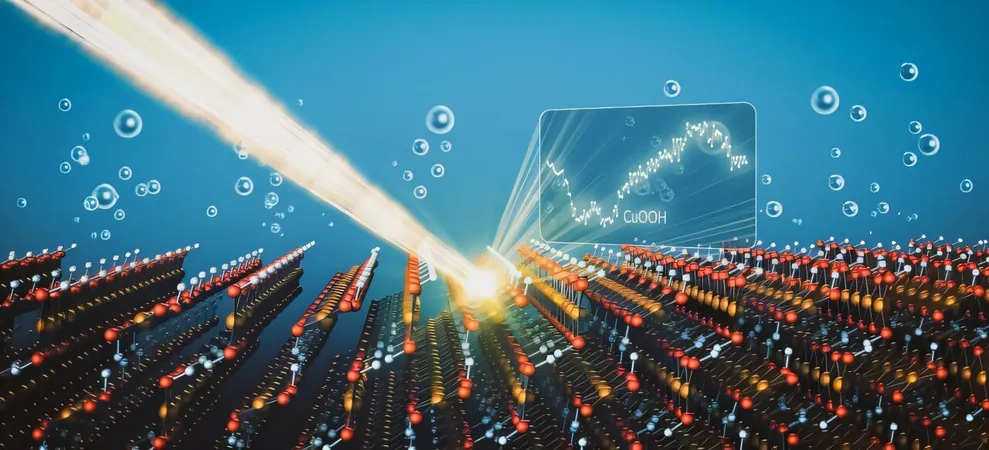
Revolutionary X-ray Technique Unveils Supercharged Copper for Water Splitting
2025-06-02
Author: Nur
Unlocking Copper's Hidden Potential
Copper is everywhere—from electrical wires to plumbing, and even in your coins. Its abundance and low cost make it a favorite for catalyzing chemical reactions, especially in breaking down water and carbon dioxide to produce clean fuels.
The Durability Dilemma
However, the ordinary copper we use isn't the most resilient catalyst available. Researchers have long been on the hunt for more durable alternatives, experimenting with oxidation—similar to how iron rusts. Back in the 1970s, chemist Marcel Pourbaix proposed that there exist particularly tough forms of oxidized copper. For decades, scientists have sought to locate these elusive compounds.
A Breakthrough Discovery
Finally, a project spearheaded by the U.S. Department of Energy's SLAC National Accelerator Laboratory has yielded success! Utilizing advanced computational techniques combined with next-gen experimental methods, a determined team of researchers, which includes experts from Lawrence Berkeley National Laboratory and Stanford University, has successfully identified a new, sturdier form of copper.
Mapping Stability and Production
In a groundbreaking study published in the Journal of the American Chemical Society, the team detailed the ideal conditions for this durable copper variant, specifically a type of copper hydroxide known as CuOOH. By applying electricity to copper electrodes immersed in an electrolyte solution, they were able to generate this material.
Computational Brilliance Meets Experimental Precision
The challenge wasn't simply to turn the system on; it required precise control over electrical voltage, acidity, and several other factors. Co-lead author Pooja Basera employed powerful computational methods to predict optimal conditions for producing the desired copper compounds. Working alongside a supercomputer at the National Energy Research Scientific Computing Center, the team matched Pourbaix's theories with remarkable accuracy.
Innovative X-ray Techniques at Work
Next, the researchers harnessed the bright X-rays from the Stanford Synchrotron Radiation Lightsource to validate their findings. Catalytic reactions occur in the surface's atomic layers, necessitating highly sensitive techniques. They relied on modulation excitation X-ray absorption spectroscopy, a cutting-edge method that creates 'structural fingerprints' of the copper electrodes, confirming the presence of the sought-after copper hydroxide.
The Formation Mystery Solved
To delve deeper, the team utilized operando Raman spectroscopy to observe molecular vibrations, helping them understand how the copper compound forms. As they increased voltage to unprecedented levels for copper studies, a new signal surfaced, matching their computational predictions and confirming the transformation to the CuOOH phase.
Transforming the Energy Landscape
These findings highlight that, in its modified form, copper can tolerate higher operating voltages, significantly boosting its durability. This breakthrough has immense consequences for electrochemical water splitting, the process used to generate hydrogen and oxygen from water—crucial for creating sustainable fuels in an energy-efficient manner, especially using solar energy.
Future Implications
Currently, copper is limited to the negatively charged electrode in water-splitting applications. However, these new discoveries may enable the metal to serve in both electrode roles, potentially replacing pricier, rarer catalysts.
A Step Towards Sustainable Catalysis
The marriage of advanced computing and cutting-edge techniques is unlocking new catalytic mechanisms. According to Dimosthenis Sokaras, a co-investigator on the project, this essential research advances the quest for stable catalysts not just in water splitting, but across various industrial applications, paving the way for a cleaner energy future.



 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 Česko (CS)
Česko (CS)
 대한민국 (KO)
대한민국 (KO)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)
 الإمارات العربية المتحدة (AR)
الإمارات العربية المتحدة (AR)