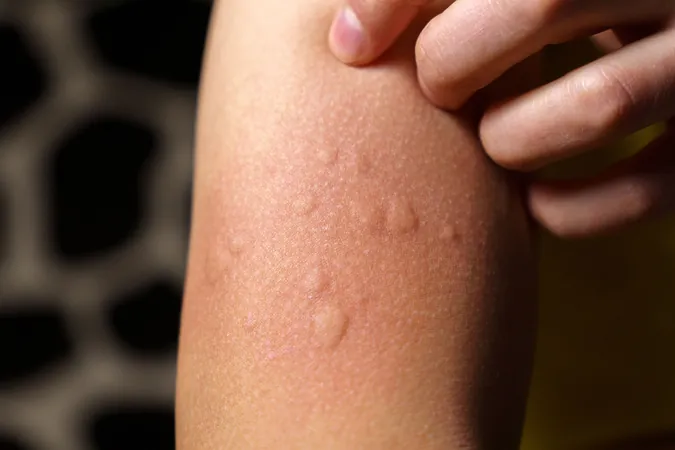
Revolutionary Treatments for Chronic Spontaneous Urticaria: A New Era Begins!
2025-04-16
Author: Arjun
Exciting Breakthroughs in Urticaria Treatment!
Recent studies reveal that innovative therapies like dupilumab, remibrutinib, and barzolvolimab offer hope for patients battling Chronic Spontaneous Urticaria (CSU), a frustrating skin allergy that often resists standard treatments. A groundbreaking review published in *Drugs* sheds light on these promising advancements.
What is Chronic Spontaneous Urticaria?
Chronic Spontaneous Urticaria is a condition marked by unpredictable outbreaks of itchy hives or swelling that can last for weeks or even months. Tragically, more than 80% of sufferers experience this relentless affliction for over a year, significantly impacting the quality of life for approximately 40% of them.
Challenges with Current Treatments
Traditional first- and second-line treatments deliver satisfactory results for only a handful of CSU patients. This lack of effective solutions is especially troubling for those with autoimmune variations of the disease, which can make up between 8% and 69% of CSU cases.
Emerging Therapies Show Promise!
Patients who don’t respond to standard treatments often exhibit characteristics of autoimmune CSU, including heightened disease activity and lower Immunoglobulin E (IgE) levels. Remarkably, some patients experience delayed responses to therapies like omalizumab, showcasing the complexity of the condition.
Dual-Action Treatments Target Multiple Conditions
Treatments focusing on more than one health issue could be a game-changer. Bruton tyrosine kinase (BTK) inhibitors are emerging as powerful contenders, showing potential not just for CSU but also for other type 2 inflammatory disorders, including asthma and atopic dermatitis.
Duplimab's Promising Results!
Dupilumab, a monoclonal antibody, has demonstrated success in interfering with the signaling pathways involved in CSU. In phase 3 trials, 30-31% of patients achieved complete relief from symptoms, featuring side effects similar to placebo.
Expanding the Arsenal Against CSU.
Remibrutinib offers a dual approach, effectively controlling mast cell activation and B cell action. Reportedly, it led to a 28-32% response rate in trials, despite some common side effects, including respiratory infections and headaches.
Barzolvolimab: A New Front in Treatment?
Barzolvolimab, with a unique mechanism targeting mast cells, is garnering attention for yielding positive results in phase 2 trials. About 38-51% of participants experienced total symptom relief, though some reported side effects like hair color changes and neutropenia.
The Future of CSU Treatments Looks Bright!
As research accelerates, the pipeline for CSU treatments is expanding rapidly, with new drugs on the horizon, including biosimilars aiming to replace established options like omalizumab. The recent FDA approval of the first respiratory biosimilar marks a pivotal moment in this journey.
Towards Tailored Treatments!
Understanding specific biomarkers in CSU patients could pave the way for personalized therapy regimens, optimizing efficacy for each individual. Future studies will be crucial in determining whether these cutting-edge treatments can change the landscape of CSU management for good.
A New Dawn for CSU Sufferers!
The possibilities presented by emerging therapies may not just alleviate symptoms, but potentially modify the disease itself, giving hope to millions suffering from this relentless condition.

 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 Česko (CS)
Česko (CS)
 대한민국 (KO)
대한민국 (KO)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)
 الإمارات العربية المتحدة (AR)
الإمارات العربية المتحدة (AR)