Revolutionary Treatment Offers Hope to Children with Rare Genetic Disorder and Averts Blindness
2024-11-12
Author: Li
Introduction
In a groundbreaking clinical trial led by experts at UCL and Great Ormond Street Hospital (GOSH), a novel treatment has emerged that holds the potential to preserve vision in children afflicted with CLN2 Batten disease, a rare genetic disorder.
Background on CLN2 Batten Disease
Published in the journal *Eye*, the study revealed that early administration of this treatment is both safe and effective in preventing severe sight loss. CLN2 Batten disease is a devastating condition that affects an estimated 30 to 50 children in the UK. Originating from a genetic mutation affecting enzyme production in the nervous system, the disease initially presents with seizures, followed by a progressive decline in motor skills, speech, and vision, culminating in cognitive decline. Without intervention, most children diagnosed with this disorder have a life expectancy of only 10 to 12 years.
Current Treatment: Brineura
Since 2019, children suffering from CLN2 Batten disease have benefited from Brineura, an enzyme replacement therapy designed to combat neurological decline through a managed access agreement. However, this agreement is set to expire in May 2025, emphasizing the urgent need for research into the treatment’s long-term effects and benefits.
Brineura is administered directly into the brain through regular infusions, effectively restoring enzyme activity and delaying disability onset. Unfortunately, this therapy does not prevent blindness, as the enzyme is unable to penetrate the blood-retina barrier, allowing ocular nerves to deteriorate, ultimately leading to vision loss.
Novel Approaches in Research
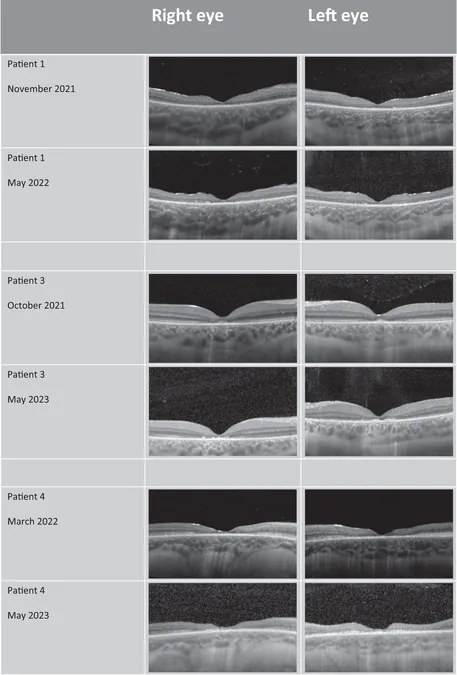
Now, researchers at the UCL Great Ormond Street Institute of Child Health and GOSH are testing a novel approach to prevent blindness. They utilize leftover Brineura samples from the brain infusion and apply them directly to the eyes. The recent study involved eight children undergoing Brineura treatment, each exhibiting significant retinal damage. The eye procedure was conducted on the same day as their routine brain infusion, under general anesthesia.
Results and Findings
Results indicated that while many participants experienced irreversible sight loss, younger children with less severe vision impairment showed promising outcomes, retaining more eyesight in the treated eye than the untreated one. Lead researcher Professor Paul Gissen emphasized the collaborative effort needed for this initiative, stating, "This work embodies a team-based approach. Historically, our Batten's patients have been vulnerable to vision loss. The developmental step of Brineura was remarkable, yet our patients continued to suffer. Partnering with the ophthalmology team, we engineered this program to enhance patient care."
Patient Testimony
One poignant testimony comes from the family of Grace, a four-year-old girl diagnosed with CLN2 Batten disease after exhibiting worrying symptoms such as slurred speech and frequent falls. Following her treatment with Brineura, Grace regained her ability to walk and began speaking again. Although initially informed that the eye treatment would be ineffective, her family was invited to participate in the new clinical trial.
Grace's mother, Izabela, expressed their gratitude, stating, "We can definitely see that the treated eye is better than the untreated one. She interacts with her favorite stories like the Gruffalo and Paddington Bear, which would have been impossible without her vision. We had been so worried about her losing her sight, and this treatment is an incredible hope for us."
Conclusion
As this research advances, it showcases a pioneering step towards not only preventing blindness in children affected by CLN2 Batten disease but also underscores the power of collaborative medical science in transforming lives. Stay tuned as we continue to follow this inspiring journey.


 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)