Revolutionary Tiny MoOx Clusters on TiO2 Nanosheets Enhance Methane Conversion
2025-07-02
Author: Siti
Breakthrough in Photocatalytic Methane Oxidation
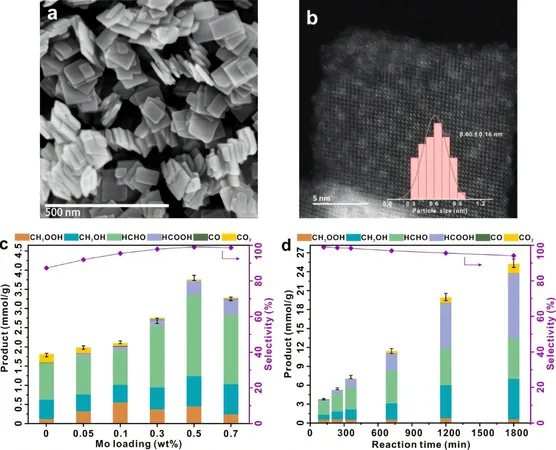
In an astonishing discovery, researchers from the Chinese Academy of Sciences have found that tiny MoOx clusters, measuring just 0.6 nanometers, when anchored onto TiO2 nanosheets, could revolutionize the process of methane oxidation. This innovative approach not only significantly boosts the production of valuable oxygenated organic compounds but also curbs the formation of CO2, a common byproduct.
The Challenge of Methane Conversion
Methane (CH4), despite its abundance, is notoriously difficult to convert into high-value products like methanol and formaldehyde due to its chemical inertness. Traditional methods often require extreme conditions, leading to over-oxidation and poor selectivity, making the quest for a more efficient process essential.
Green Solutions through Photocatalysis
Photocatalysis emerges as a promising and eco-friendly alternative, utilizing solar energy to drive reactions. However, enhancing both reactivity and selectivity in these processes has proven challenging. While noble metal catalysts, like gold, can improve performance, their prohibitive costs hinder widespread implementation.
A Game-Changing Catalyst Design
To overcome these limitations, this groundbreaking study presents a TiO2-based photocatalyst enhanced with MoOx clusters. The optimal catalyst, with just a 0.5% MoOx loading, achieved an organic oxygenate yield of 3.8 mmol/g in just two hours, boasting an impressive selectivity of nearly 100%. Add to that an apparent quantum yield of 13.3% at a wavelength of 365 nm, and it sustained over 95% selectivity for an astounding 1,800 minutes!
Unveiling the Mechanism of Action
Through in situ Electron Paramagnetic Resonance (EPR) and Nuclear Magnetic Resonance (NMR) analyses, the researchers unveiled how MoOx clusters activate O2, forming surface-active species that facilitate the activation of methane’s carbon-hydrogen bonds. This crucial step stifles the generation of undesirable hydroxyl and superoxide radicals, which are often responsible for over-oxidation.
Fostering Efficiency and Precision
Additionally, the photogenerated electrons play a vital role by reducing Mo species. In the presence of water, this process aids in the desorption of formaldehyde from the catalyst surface, ensuring that the valuable product does not undergo further unwanted oxidation.
A Bright Future for Sustainable Methane Utilization
This pioneering research not only opens new avenues in photocatalytic methane conversion but also highlights the potential of inexpensive, non-noble metal catalysts in achieving efficient, selective, and sustainable chemical transformations — a step closer to harnessing the full potential of methane as a valuable resource.
 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 Česko (CS)
Česko (CS)
 대한민국 (KO)
대한민국 (KO)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)
 الإمارات العربية المتحدة (AR)
الإمارات العربية المتحدة (AR)