Revolutionary Synthetic mRNA Therapy Offers Hope Against Metastatic Cancer without Harmful Side Effects!
2025-03-17
Author: Jia
Introduction
Metastatic cancer, where cancer cells spread to various organs, is one of the leading causes of cancer-related fatalities worldwide. Conventional treatments, including surgery, chemotherapy, and radiation, often become less effective when the disease progresses beyond its primary site. However, a team of innovative scientists from Shinshu University School of Medicine has made a groundbreaking advance by developing a synthetic messenger RNA (s-mRNA) therapy that can bolster the immune system's ability to combat metastasizing cancer cells.
The Challenge of Metastatic Cancer
Cancer remains a critical health challenge globally, with rising incidence rates presenting an alarming trend. The process of metastasis involves cancer cells evading the immune system’s responses, leading to widespread disease and decreased survival rates. Notably, these cells employ complex mechanisms to suppress immune functions, reprogramming crucial cells like natural killer (NK) cells and cytotoxic T lymphocytes (CTLs) to foster an immunosuppressive environment instead of attacking tumors.
Groundbreaking Developments in s-mRNA Therapy
With the expertise of Professor Sachie Hiratsuka, Associate Professor Takeshi Tomita, and Professor Yoshihito Ueno, the research team unveiled an innovative s-mRNA that can be injected into the bloodstream, effectively enhancing the immune response against cancer targets. This significant discovery was published in the prestigious journal *Nature Communications* on February 25, 2025, marking a potential turning point in cancer treatment.
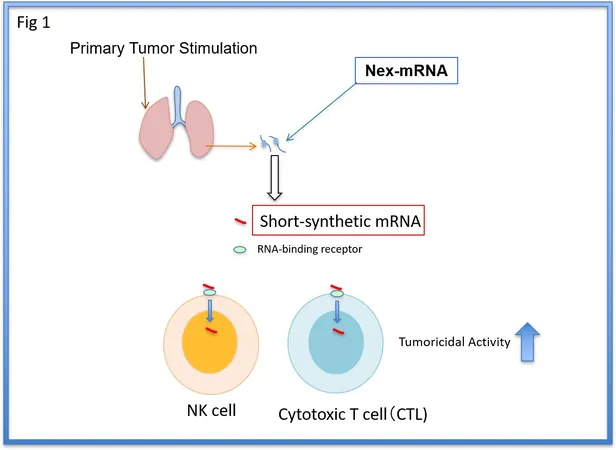
Mechanism of Action of the Synthetic mRNA
Their synthesized mRNA is derived from a naturally occurring IL1β-mRNA, identified in mice prior to the onset of metastasis. By binding to ZC3H12D, an RNA-binding protein found on NK cells, this synthetic version activates these immune cells. However, because natural IL1β-mRNA is prone to quick degradation, the researchers modified the structure to increase its stability and effectiveness when administered to the body.
Experimental Results
In their experiments, the team found that the mRNA could remain intact in human and mouse serum for up to 48 hours, allowing it to effectively bind to immune cells, enhancing their capabilities to attack tumors. Crucially, this process avoids triggering a cytokine storm—a potentially life-threatening overreaction of the immune system.
Impact on Tumor-Bearing Mice and Human Cells
In trials with tumor-bearing mice, who were implanted with breast and colon cancer cells, administering just three small doses of s-mRNA led to a significant reduction in metastatic cells within the lungs. Remarkably, immune cells remained active long after the treatment, demonstrating sustained tumor-fighting abilities even against residual cancer cells post-surgery. Furthermore, the results showed promise beyond animal models. In laboratory settings, when the s-mRNA was applied to weakened immune cells obtained from colon cancer patients, the cells exhibited a remarkable capability to kill up to 70% of cancer cells, even in individuals undergoing anticancer therapies.
Future Prospects and Conclusion
This therapeutic approach also presents the opportunity to combine with existing cancer treatments, such as anti-PD1 antibodies, potentially leading to enhanced patient outcomes. One of the most compelling features of the s-mRNA therapy is its ability to be administered in multiple doses without causing harmful inflammatory side effects, making it a versatile option in the fight against metastatic cancer. As the scientific community eagerly anticipates further developments in this research, the implications of this synthetic mRNA therapy could redefine treatment strategies for cancer patients, offering newfound hope in the battle against a disease that continually poses grave challenges. With ongoing studies, advancements in this area could mean the difference between life and death for countless individuals facing metastatic cancer.


 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 Česko (CS)
Česko (CS)
 대한민국 (KO)
대한민국 (KO)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)
 الإمارات العربية المتحدة (AR)
الإمارات العربية المتحدة (AR)