Revolutionary Sub-5 nm Ru-Based Alloy Catalysts Set to Transform Water Splitting Technology
2025-03-21
Author: Yu
Groundbreaking Breakthrough
A groundbreaking breakthrough has emerged from the Hefei Institutes of Physical Science, led by Prof. Liang Changhao of the Chinese Academy of Sciences, who has unveiled an innovative method that could revolutionize hydrogen production. The research, which has found its place in the prestigious journal Advanced Science, is focused on the development of carbon nanotube (CNT)-supported intermetallic RuM (with M representing Cu, Rh, or Pd) alloys featuring nanoparticles (NPs) that are impressively less than 5 nanometers in size.
Challenges in Synthesis
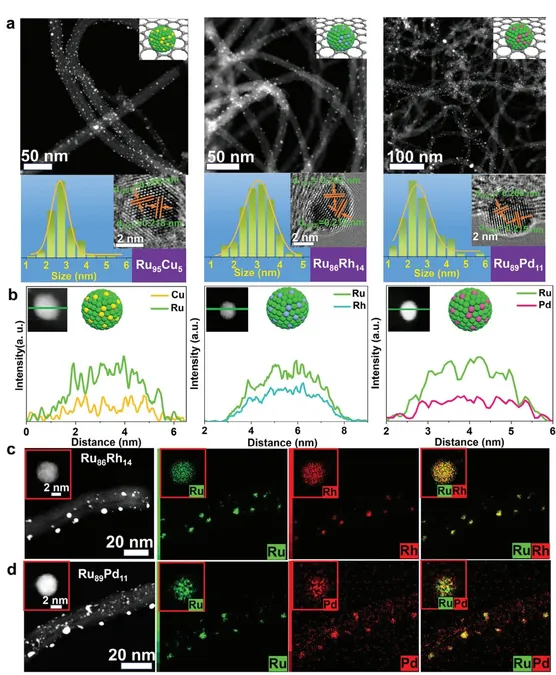
These sub-5 nm Ru-based alloys have sparked considerable interest in the field of electrochemical catalysis, particularly for water splitting. Previous challenges in their synthesis have primarily stemmed from thermodynamic immiscibility, which has hindered the application of a universal preparation process. However, this study introduces a solution through a cutting-edge technique known as nanosecond laser ultrafast confined alloying (LUCA). This method adeptly navigates the complex transition from immiscible to miscible states, paving the way for the creation of CNT-supported bimetallic RuM alloy NPs.
Exceptional Electrocatalytic Performance
Among the catalysts synthesized, the Ru95Cu5/CNTs variant exhibited extraordinary electrocatalytic performance during the alkaline hydrogen evolution reaction (HER). It achieved an astonishingly low overpotential of merely 17 mV and recorded a Tafel slope of 28.4 mV dec⁻¹ at an operational current density of 10 mA cm⁻². These figures not only suggest exceptional efficiency but also hint at the potential for real-world application in sustainable energy solutions.
Remarkable Stability and Comparisons
What stands out even further is the catalyst’s remarkable stability; it maintained its efficiency over 5,000 cycles of cyclic voltammetry. In head-to-head comparisons, Ru95Cu5/CNTs outshined several other catalysts, including the LUCA-synthesized Ru86Rh14/CNTs, Ru89Pd11/CNTs, and even established commercial benchmarks like the 20% Pt/C catalysts, which are frequently used in the industry.
Performance Metrics
Furthermore, performance metrics showed that the Ru95Cu5/CNTs catalyst surpassed others, including RuRh particles synthesized via wet chemistry and RuCu/CNTs developed through flash Joule heating methods. For instance, these alternatives exhibited higher overpotentials, with RuRh showing 25 mV and RuCu at 39 mV.
Economic Implications
The appeal of this research extends beyond performance; the strategic alloying of cost-effective non-noble metals like copper with varying atomic ratios in RuCu alloys presents an exciting path toward economical, large-scale hydrogen production. As the world grapples with the urgent need for sustainable energy solutions, the implications of this study are profound.
Conclusion
In summary, this research not only indicates a leap forward in the efficiency and cost of hydrogen production through water splitting but also highlights the immense potential of LUCA as a transformative tool in the quest for new classes of HER catalysts. This breakthrough opens avenues for further exploration and development, promising a greener and more sustainable future.





 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 Česko (CS)
Česko (CS)
 대한민국 (KO)
대한민국 (KO)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)
 الإمارات العربية المتحدة (AR)
الإمارات العربية المتحدة (AR)