Revolutionary Study Using High-Resolution IR Spectroscopy Unveils Hidden Secrets of Hydrogen Sulfide Bonding!
2024-11-12
Author: Mei
In a surprising twist of chemistry, it turns out that water and the notorious stink of hydrogen sulfide (H2S) share more than just a symbolic connection. While the ice-laden refreshment in your favorite drink and grandma’s infamous egg salad smell might appear worlds apart, a closer look reveals intriguing similarities at the molecular level.
The research team from the Bochum Cluster of Excellence "Ruhr Explores Solvation" (RESOLV) in Germany has pulled back the curtain on the enigma of hydrogen bonding in H2S, a molecule often overlooked in comparison to its abundant counterpart, H2O. Led by Professor Martina Havenith and published in *Nature Communications* on November 5, 2024, their study sheds light on how H2S behaves in ways that could reshape our understanding of both fundamental chemistry and biological processes.
H2S is not just a mere nuisance with its foul odor; it is a primordial molecule found in the vastness of the interstellar medium and plays crucial roles in various biological systems across species. Despite ongoing infrared studies, many questions about H2S’s interactions remained unresolved.
Unconventional Techniques Unravel Chemistry Mysteries
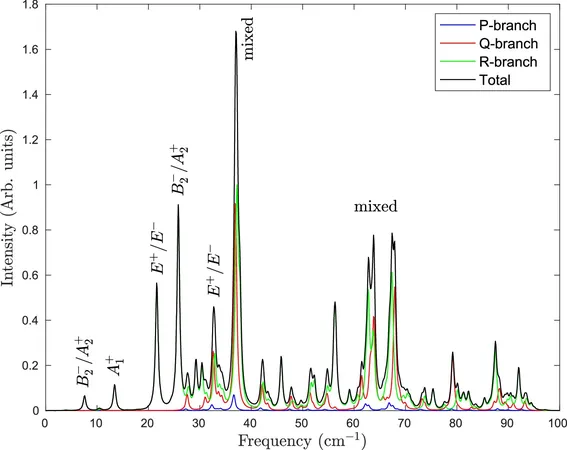
What makes this research groundbreaking is the use of high-resolution infrared (IR) spectroscopy conducted in an innovative environment — superfluid helium nanodroplets. The Bochum researchers, including Svenja Jäger, Philipp Meyer, and Jai Khatri, embedded H2S molecules within these unique droplets, allowing for remarkable control over their experimental conditions.
Superfluid helium presents a unique laboratory, thanks to its extraordinary properties that include high thermal conductivity, transparency across a wide spectral range, and negligible interaction with the encapsulated molecules. These attributes enabled the researchers to closely examine the interplay between two H2S molecules without interference from outside influences, allowing for unprecedented clarity in detecting their vibrational movements and energy states.
Major Discoveries and Implications for Science
The findings revealed not only the vibrational activities of the H2S dimer but also its rotational dynamics and the concept of tunneling splitting — a major breakthrough that explains energy transitions at a quantum level. Researchers found exciting parallels between H2S and water when one molecule is excited, suggesting that under the right conditions, H2S can replicate the hydrogen bonding characteristics seen in water.
Additionally, the team re-evaluated existing vibrational signals noted by prior chemical inquiries and established rigorous benchmarks for computational methods that predict molecular interactions. These methods are crucial for understanding how different molecules behave and can have far-reaching implications in fields ranging from astrobiology to materials science.
A New Chapter in Molecular Chemistry
The insights gained from this study not only provide a deeper understanding of hydrogen bonding in H2S but also set the groundwork for future research into its role in larger biochemical processes and its potential applications in various industrial sectors. As scientists continue to unravel the mysteries of this simple yet fundamental molecule, we may soon discover new pathways for innovation that harness its unique properties.
Stay tuned as we delve deeper into the fascinating world of molecular chemistry, where every discovery can turn the tides of science!




 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)