Revolutionary Study Unveils Fast-Track Lipid Transport Directly to the ER, Outshining Traditional Vesicular Methods!
2025-09-02
Author: Nur
Groundbreaking Research from Max Planck Institute
A striking new study led by the Max Planck Institute of Molecular Cell Biology and Genetics has unveiled a game-changing finding: direct lipid transfer from the plasma membrane to the endoplasmic reticulum (ER) is astonishingly rapid, surpassing the traditional, slower vesicular trafficking methods. This research has culminated in a comprehensive map that details retrograde lipid movement in living cells.
The Lipid Landscape of Cells
Within our cells, diverse lipid species perform critical duties, with each organelle membrane housing specific lipids suited to distinct cellular tasks. However, the intricacies of how these vital lipids travel between organelles—an essential process for cellular function—have remained largely unclear due to limitations in live-cell imaging technology.
Decoding Lipid Traffic: From Creation to Distribution
Lipids are synthesized in the ER and then dispatched towards the plasma membrane, where they might be recycled back to the ER or transported to lysosomes, peroxisomes, and mitochondria for breakdown. While earlier studies monitored some aspects of this journey via metabolic labeling, less clarity existed around retrograde pathways, aside from notable exceptions like sphingomyelin.
Innovative Methodology Reveals the Unseen
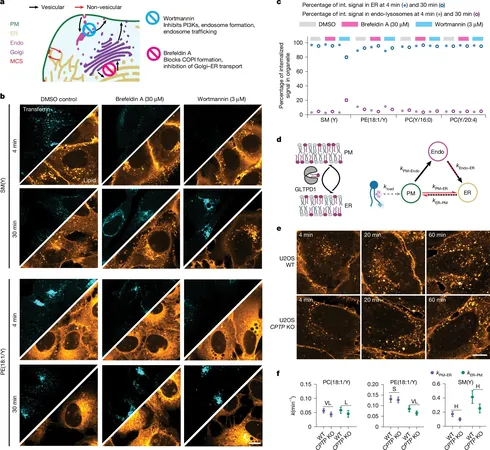
Published in the prestigious journal Nature, the research titled "Quantitative imaging of lipid transport in mammalian cells," harnessed cutting-edge techniques. Researchers utilized time-resolved fluorescence imaging alongside ultra-high-resolution mass spectrometry and mathematical modeling to analyze individual lipid species.
Using U2OS cells for loading and imaging, the team employed a cyclodextrin carrier to insert bifunctional lipids into the plasma membrane—captured using ultraviolet light for visualization. The probes effectively monitored both the location and metabolic fate of various lipids, including phosphatidylcholine and sphingomyelin.
Startling Findings on Transport Speed
The results were eye-opening: lipids like phosphatidylethanolamine and polyunsaturated phosphatidylcholine zoomed into the ER almost immediately after loading. In contrast, saturated phosphatidylcholine and sphingomyelin lingered longer at the plasma membrane. Astonishingly, direct transport outpaced vesicular movements by an incredible eleven-fold!
Among the phosphatidylcholine group, unsaturated species exhibited transport speeds up to seven times faster than their saturated counterparts. Notably, the position of chains on lipid molecules also influenced speed, with sn-2 chains traveling twice as fast as those on sn-1.
A New Era of Lipid Biology Insights
Interestingly, blocking vesicular transport did not hinder the early delivery of lipids to the ER, although sphingomyelin began to accumulate in endosomes over time. Disruptions in specific transport proteins significantly impacted lipid delivery dynamics.
Furthermore, transport proved to be significantly faster than metabolic processes, with speeds exceeding chemical conversions by ten to sixty times, suggesting a finely-tuned relationship between the two.
Implications for Future Research
This revolutionary study underscores non-vesicular transport's dominance in organelle lipid distribution and positions ATP-dependent lipid flipping as a key mechanism for directed movement. The research not only paves the way for understanding lipid metabolism but also opens doors for innovations in target discovery and lipid-storage biology.
Ultimately, this detailed atlas of lipid transport sets a foundation for future studies, promising an exciting future in cellular biology.

 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 Česko (CS)
Česko (CS)
 대한민국 (KO)
대한민국 (KO)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)
 الإمارات العربية المتحدة (AR)
الإمارات العربية المتحدة (AR)