Revolutionary Study Reveals Why Graphite Can Form Instead of Diamond Under Extreme Conditions!
2025-07-09
Author: Rajesh
You might be surprised to learn that the graphite in your pencil could have been the dazzling diamond adorning your mother’s finger! But how does this happen? A groundbreaking study is shedding light on this fascinating phenomenon.
Understanding how molten carbon transitions into either graphite or diamond is crucial for fields ranging from planetary science to nuclear fusion. However, observing this crystallization process in real time poses significant challenges due to its rapid occurrence under extreme conditions.
In a stunning new study published in *Nature Communications*, researchers from the University of California, Davis and George Washington University used advanced computer simulations to explore the conditions under which molten carbon crystallizes into graphite or diamond, mimicking the intense pressures and temperatures found deep within Earth.
Against all odds, the team's findings upend conventional wisdom about diamond formation and clarify the inconsistencies seen in previous experimental results regarding carbon's phase behavior.
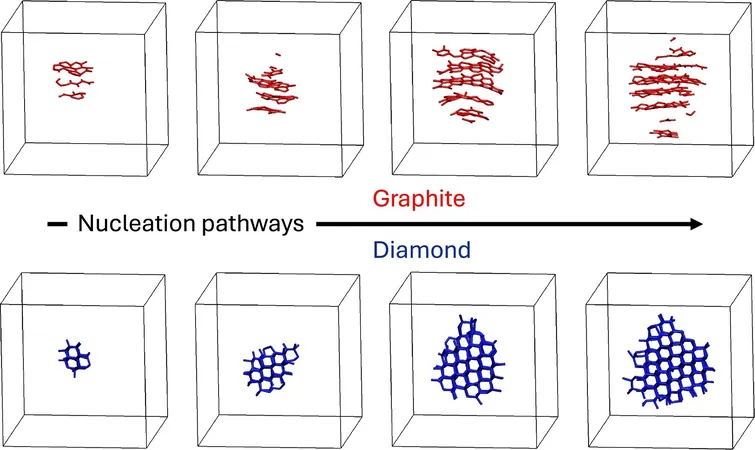
Utilizing state-of-the-art machine learning techniques, the researchers uncovered that molten carbon displays much more complicated crystallization behavior than previously recognized. Most astonishingly, they discovered that graphite—the seemingly mundane form of carbon—can inadvertently form even within conditions typically suitable for diamond creation, essentially "hijacking" the diamond formation process.
Simulating Earth’s Extreme Conditions
The research team was able to provide a detailed atomistic view of how this transformation occurs, examining models at pressures between 5 to 30 gigapascals (GPa) as molten carbon cooled from a blazing 5,000 to 3,500 Kelvin (K). According to researcher Donadio, such extreme conditions can be mirrored in laser heating experimental setups.
While they anticipated the outcome would be glassy carbon following the rapid cooling, the team was surprised to witness spontaneous crystallization. At elevated pressures, the molten carbon turned into diamond, while lower pressures favored graphite formation.
"This unexpected outcome is significant because simulating crystallization is usually much more intricate," Donadio shared. "We were particularly amazed to see graphite form spontaneously at pressures up to 15 GPa, where diamond is normally expected to be stable."
Understanding the Path of Least Resistance
This intriguing behavior aligns with Ostwald's step rule, which suggests that crystallization can sometimes pass through intermediate metastable states rather than proceeding directly to the most stable species. The researchers revealed that graphite serves as a stepping stone toward diamond since its structure closely resembles the density and bonding patterns of liquid carbon.
"Liquid carbon finds it easier to transform into graphite first, despite diamond being the ultimately more stable phase under these conditions," noted co-author Tianshu Li, a civil and environmental engineering professor at George Washington University. "It's nature taking the path of least resistance."
Insights into Crystallization Dynamics
Through their simulations, the researchers delineated the molecular structures of liquid carbon as it transformed into graphite and separately into diamond. Graphite crystallized in elongated, column-like shapes, while diamond formed through compact arrangements.
This research addresses longstanding discrepancies in high-pressure carbon experiments, offering a new lens through which to interpret previously puzzling results.
The findings carry significant implications: they help explain the rarity of natural diamond formation and contribute to our understanding of the deep carbon cycle, crucial for Earth’s climate and geology over millions of years. Furthermore, insights into these crystallization pathways could revolutionize diamond manufacturing techniques, especially for precision applications like quantum computing.
"Crystallization is a fundamental process for technology, and diamonds are invaluable materials," said Donadio. "Our work elucidates the presence of graphite in situations where it might not traditionally be expected."
This groundbreaking research was co-authored by Margaret L. Berrens, Wanyu Zhao, and Shunda Chen.


 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 Česko (CS)
Česko (CS)
 대한민국 (KO)
대한민국 (KO)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)
 الإمارات العربية المتحدة (AR)
الإمارات العربية المتحدة (AR)