Revolutionary Study Reveals Speed, Not Strength, Is Key to Cell Membrane Binding for Biomaterials
2025-09-19
Author: Nur
Molecular Motion: The Game-Changer in Biomaterials Binding
A groundbreaking study led by Professor Dr. Shikha Dhiman from Johannes Gutenberg University Mainz has uncovered a shocking truth: the speed of molecular movement in cell membranes is the true catalyst for whether these membranes successfully bind to biomaterials, rather than the previously assumed binding strength.
The Promise of Tissue Engineering Hasn't Been Fully Realized
Tissue engineering, a field that holds immense promise for growing skin and organs from stem cells to facilitate improved wound healing and transplants, has seen progress but hasn't met the high expectations set two decades ago. This lag is primarily due to stem cells failing to bind effectively to the host materials as theory suggests they should.
Scientists Unlock the Mystery of Cell-Material Interaction
The international research team's findings, published in the journal PNAS, could change the future of biomaterials. Dhiman notes, "It's not just the strength of the interaction that matters; it's the speed at which these binding partner molecules move. This revelation is key for developing more effective biomaterials."
From Theory to Practice: Rethinking Traditional Approaches
Biotechnologists typically place stem cells on gel-like matrices to guide their behavior. Traditionally, the assumption was that simply adding strong-binding molecules—known as ligands—would ensure effective bonding to cell receptors. However, this assumption often fell flat in practical settings.
Challenging Conventional Wisdom: Speed is Everything
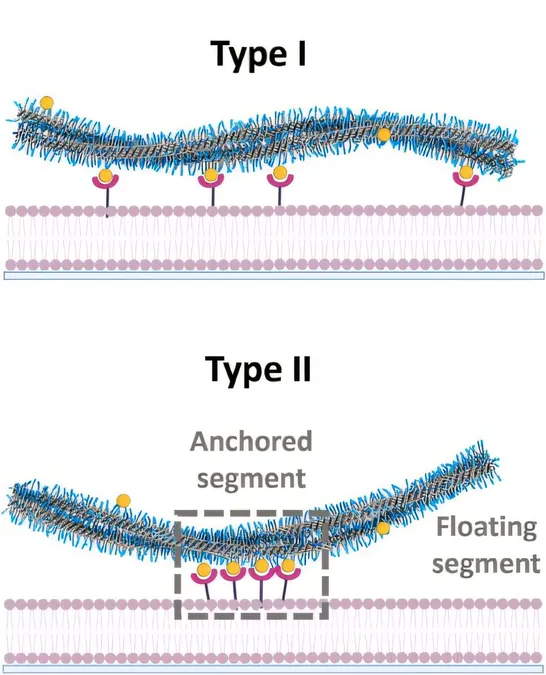
Unlike many researchers who focus on strengthening the matrix material, Dhiman and her collaborator, Professor Bert Meijer from Eindhoven University of Technology, investigated the core interaction at a much finer scale: the bond between individual fibers and model cell membranes. "Previous studies stressed the importance of connection strength, but we found that a model cell membrane's ability to bind to the fibers is primarily determined by the speed at which molecules in both the model cell membrane and fibers move," Dhiman explained.
This means that when the ligand speed in the fiber matches that of the receptors in the model cell membrane, stronger interactions occur. "Even the weakest bonds can lead to significant interactions if their speeds align," she adds.
Innovative Techniques Reveal New Insights
Employing super-resolution microscopy, the researchers were able to observe the dynamic behavior of individual receptors and ligands. By focusing on single fibers instead of bulk gel, they gained clarity on these essential interactions. Dhiman emphasized, "When movement speeds match, these molecules tend to cluster together, which facilitates binding more effectively than individual molecules could."
The possibility of entire groups of receptors and ligands coming together makes even weak bonds sufficient for successful binding.
A Bright Future for Medicine?
These findings could potentially revolutionize not only the field of tissue engineering but also other critical medical areas such as immunotherapies and tailored drug delivery systems. Imagine delivering medication directly to its target to enhance efficacy and mitigate side effects—this study paves the way for such advancements in modern medicine.




 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 Česko (CS)
Česko (CS)
 대한민국 (KO)
대한민국 (KO)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)
 الإمارات العربية المتحدة (AR)
الإمارات العربية المتحدة (AR)