Revolutionary Study Reveals Link Between Gut Bacteria and Rheumatoid Arthritis
2025-05-02
Author: Nur
Gut Microbes and Autoimmune Diseases: A Surprising Connection
In a groundbreaking study, researchers have unveiled how "good" gut bacteria might play a crucial role in the onset of rheumatoid arthritis (RA) and possibly other autoimmune diseases. By tracking a unique immune cell in mice, scientists have connected these beneficial microbes to a worrying twist in the body’s immune response.
The Role of T Cells: A Double-Edged Sword
Since a pivotal study in 2016, it has been understood that specific commensal bacteria—harmless critters living in our gut—can trigger the production of a particular T cell type linked to autoimmune diseases. This unexpected relationship highlights the gut’s influence on T cells, which possess remarkable plasticity, allowing them to adapt to different environments, leading to potential chaos within the body's immune system.
Hsin-Jung Joyce Wu, a leading author from The Ohio State University, emphasizes the alarming implications of T cell plasticity in autoimmune diseases. "This is the first instance where we see T cell plasticity in the gut causing systemic effects that escalate autoimmune issues."
The Human Element: Relevance to RA Patients
The study’s implications extend to humans, as many genetic markers found in the mouse models also appear in cells from patients suffering from rheumatoid arthritis. With an estimated 18 million people worldwide afflicted by RA, understanding these mechanisms could pave the way for new treatment strategies.
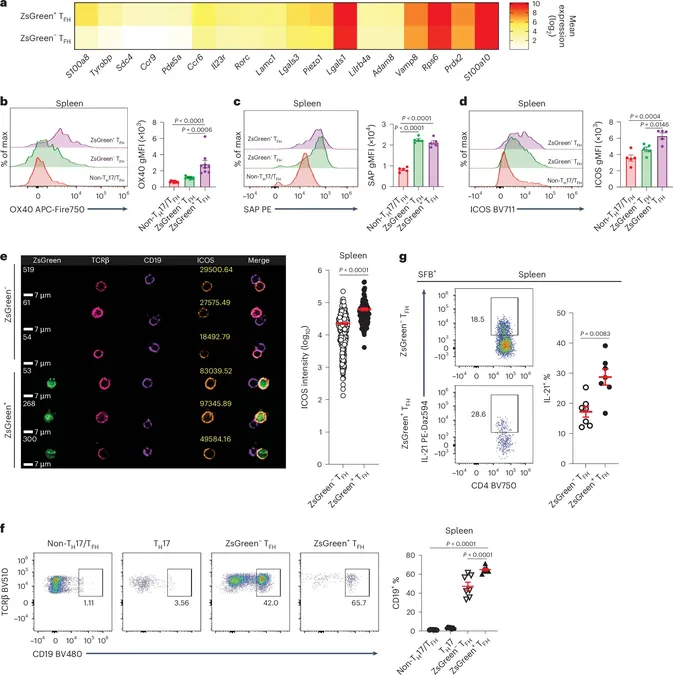
Unraveling the Abnormal T Follicular Helper Cells
The research identifies a specific rogue T cell called TFH17, which exhibits characteristics of both TFH and TH17 cells. Unlike ordinary TFH cells, which stay put, TFH17s can migrate throughout the body, intensifying the body’s inflammatory response. This migratory ability allows these cells to worsen RA symptoms, creating a troubling cycle.
A Closer Look at the Gut Connection
After their initial findings, Wu's team discovered that these aberrant TFH cells stem from Peyer’s patches in the gut, influenced by normally non-threatening segmented filamentous bacteria. These innocent-seeming microbes are surprisingly pivotal in reprogramming T cells, potentially escalating the risk of autoimmune diseases.
Tracking Mobility: A Novel Approach
In a notable experiment, Wu’s team employed fluorescent tagging techniques to visualize the movement of these cells from the gut to other parts of the body. Their findings were eye-opening; the research revealed that these mobile TFH17 cells considerably enhance B cell activity, making them particularly dangerous in the context of RA.
Implications for Future Treatments
To gauge the impact of these abnormal cells on arthritis development, the researchers injected genetically susceptible mice with either conventional TFH cells or a mix including around 20% TFH17 cells. Astonishingly, even a small percentage of the aberrant cells surged RA symptoms by nearly five times compared to controls.
This study not only sheds light on the intricate link between gut bacteria and autoimmune responses but also hints at a similar underlying mechanism in human cases of RA. As the research is published in *Nature Immunology*, it opens a new chapter in understanding and combating autoimmune diseases.

 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 Česko (CS)
Česko (CS)
 대한민국 (KO)
대한민국 (KO)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)
 الإمارات العربية المتحدة (AR)
الإمارات العربية المتحدة (AR)