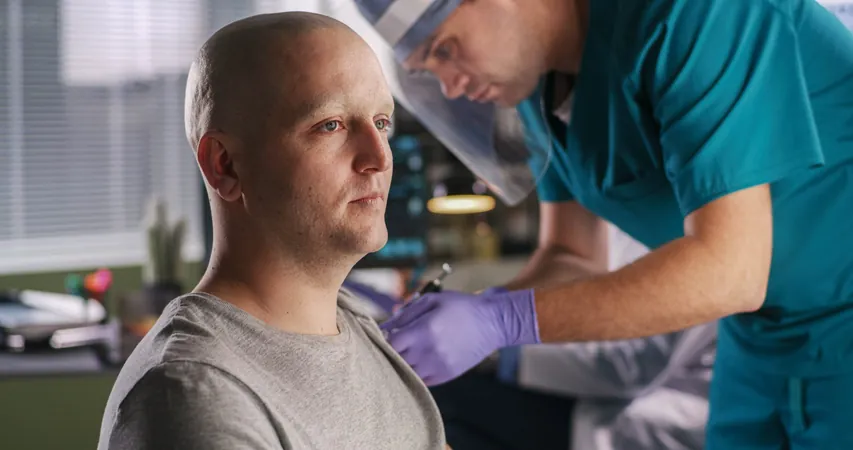
Revolutionary Skin Cancer Vaccine Trials to Kick Off in the NHS
2025-04-14
Author: John Tan
Breakthrough for Advanced Skin Cancer Patients
In a significant leap forward for cancer treatment, patients battling advanced skin cancer in England are about to be fast-tracked into groundbreaking trials for a new vaccine. This innovative therapy aims to enhance the immune system’s ability to recognize, attack, and retain a ‘memory’ of cancer cells, offering hope for preventing future recurrences.
Pioneering NHS and Scancell Partnership
The NHS has joined forces with UK life-sciences powerhouse Scancell to streamline patient access to these trials. Initial registrations include seven trial sites, with the first patient referrals expected as soon as May 2025.
Cancer Vaccine Launch Pad Expands Horizon
This exciting development is a part of NHS England’s trailblazing Cancer Vaccine Launch Pad (CVLP)—a world-first initiative designed to expedite patient access to pioneering vaccine studies at local hospitals. The CVLP has previously facilitated thousands of patients in accessing personalized vaccine trials for bowel cancer, successfully fast-tracking over 350 patients for consideration.
Expert Insights on New Vaccine's Potential
Professor Peter Johnson, NHS National Cancer Director, expressed immense optimism: "Skin cancer is profoundly devastating, but cancer vaccines could revolutionize treatment for patients in the UK and beyond, saving countless lives. Expanding our program means more patients can discover new vaccine options that could significantly alter their prognosis."
Spotlight on Melanoma: A Growing Threat
Melanoma, now the fifth most common cancer in the UK, constitutes about 4% of all new cancer cases. Standard treatments are effective for approximately half of melanoma patients, but those who don’t respond face a heightened risk of disease progression.
Introducing iSCIB1+: The Next Generation Vaccine
The new DNA vaccine, dubbed iSCIB1+ (Immunobody®), uniquely targets biomarkers specific to melanoma tumors, triggering a T-cell response that identifies and dismantles cancer cells. This immune ‘memory’ could play a critical role in preventing the disease from recurring.
What to Expect: The SCOPE Phase II Trial
Coordinated by the Southampton Clinical Trials Unit, the phase II trial, known as SCOPE, aims to broaden enrollment, with plans to recruit dozens of patients by October. To qualify for the trial, advanced melanoma patients who have not received treatment must undergo a blood test assessing their tissue type, which holds important information about their immune system.
A Personal Stake in Progress
Karin Smyth, Minister of State for Health and a skin cancer survivor herself, shared her insights: "Each treatment advancement brings renewed hope to patients and families. Our collaborative effort through the Cancer Vaccine Launch Pad could redefine approaches to treating advanced melanoma, ensuring wide access to potential lifesaving innovations."
Promising Path Ahead for Cancer Research
Professor Gareth Griffiths from the Southampton Clinical Trials Unit remarked on the importance of the CVLP’s expansion, noting their commitment to boosting patient referrals and providing access to potentially life-saving treatments. Dr. Iain Foulkes from Cancer Research UK highlighted the vital role of cancer vaccine research, stressing the need for diverse research avenues to prolong lives and enhance quality of life.

 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 Česko (CS)
Česko (CS)
 대한민국 (KO)
대한민국 (KO)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)
 الإمارات العربية المتحدة (AR)
الإمارات العربية المتحدة (AR)