Revolutionary Single-Atom Catalysts Pave the Way for Greener Chemical Production
2025-04-14
Author: Mei
A Game-Changer in Chemical Synthesis
Chemists at the National University of Singapore (NUS) have unveiled a groundbreaking approach to catalysis with the development of "artful single-atom catalysts" (ASACs). Utilizing an innovative "anchoring-borrowing" technique alongside advanced facet engineering, these catalysts are set to transform the landscape of sustainable chemical and pharmaceutical synthesis.
Unlocking the Power of Single-Atom Catalysts
Single-atom catalysts (SACs) have been the talk of the scientific community due to their efficiency in maximizing atomic use and establishing highly active reaction sites. They merge the advantages of traditional and modern catalytic methods, promising enhanced performance in fine chemical manufacturing.
The Challenge of Stability and Reactivity
Traditionally, the materials designed to stabilize metal atoms often compromise their reactivity. Strong bonds can hinder the metal's ability to participate in complex reactions, especially in multi-step processes like cross-coupling, which are crucial in pharmaceuticals.
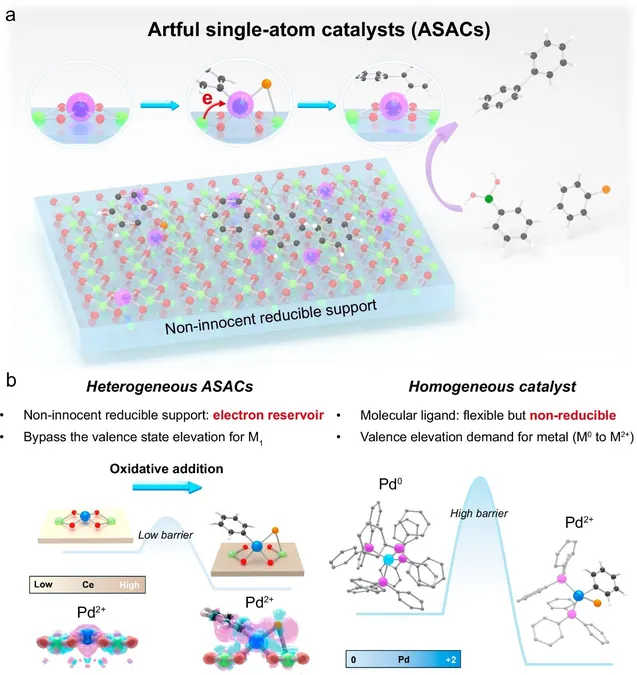
A Revolutionary Strategy: Anchoring-Borrowing
Under the leadership of Associate Professor Lu Jiong, the NUS team introduced the "anchoring-borrowing" method, which involves securing single metal atoms on specific sites of metal oxide surfaces. This process allows these surfaces to "borrow" oxygen atoms, enabling the metal to remain reactive without the drawbacks of rigid electronic structures.
High Performance with Difficult Chemicals
Using cerium oxide (CeO2) as a support, the researchers discovered that the newly devised Pd1-CeO2 ASAC excels in challenging reactions involving aryl chlorides and complex compounds. This catalyst not only surpassed traditional options in yield and stability but also established a new benchmark for catalysis.
Versatile and Scalable: The Future of Catalysts
The ASACs' ability to produce high yields quickly and in substantial quantities presents significant potential for the large-scale manufacturing of pharmaceutical ingredients. They also demonstrate flexibility for various reactions, including the Heck and Sonogashira transformations.
Groundbreaking Research and Future Prospects
Through a combination of theoretical and experimental research, the NUS team determined that ASACs dynamically adjust the structure of the palladium atom, using CeO2 as an electron reservoir. This stabilization lowers the energy barrier for reactions, maintaining the palladium's effectiveness throughout.
A Greener Path for Chemistry
According to Associate Professor Lu, "This novel concept of heterogeneous ASACs presents a much greener solution to the longstanding issue of oxidative addition. Our strategy surpasses the limitations of existing catalysts, holding immense promise for sustainable chemical and pharmaceutical production." Looking forward, the team aims to broaden this innovative approach to include a range of metals, potentially enhancing the efficiency of more abundant, non-precious metals in cross-coupling reactions.


 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 Česko (CS)
Česko (CS)
 대한민국 (KO)
대한민국 (KO)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)
 الإمارات العربية المتحدة (AR)
الإمارات العربية المتحدة (AR)