Revolutionary Research Reveals How Microwaves Can Delay Chemical Reactions!
2025-03-24
Author: Arjun
Introduction
In an intriguing breakthrough in the realm of chemistry, researchers from the Swiss Federal Institute of Technology (ETH) Zurich have shown that microwaves can not only hasten chemical reactions but can also cool down molecular activity, leading to the suppression of certain reactions. This unprecedented finding may change the way we understand microwave interactions within chemical processes.
Traditional Understanding of Chemical Reactions
Traditionally, chemical reactions are accelerated through heat, as outlined by Arrhenius’ law, which states that raising temperatures increases molecular energy—allowing more molecules to overcome the activation barrier and react. Microwaves have been employed as a heating method for many years, promoting accelerated reactions in various chemical syntheses. However, the implications of this new research extend far beyond mere heating.
The Groundbreaking Experiment
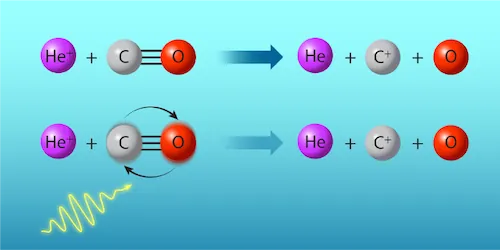
Valentina Zhelyazkova and her team from ETH Zurich conducted a groundbreaking experiment focusing on the reaction between positively charged helium ions (He+) and carbon monoxide (CO) molecules. By carefully controlling microwave pulses, they could shift the CO molecules into a less reactive state—essentially tuning the reaction's rate to either boost or suppress reactions as desired.
Understanding Capture Theory
The scientists operated under the principles of capture theory, which suggests that the speed of reactions depends heavily on the rotational states of the molecules involved. Using specific techniques to create separate beams of He atoms and CO molecules, they were able to make intricate adjustments to the state of the CO molecules using precisely-timed microwave energy. This allowed the microwave pulses to excite the CO only partially, demonstrating a remarkable control over molecular reactivity.
Future Applications and Implications
What’s more, these groundbreaking findings open exciting avenues for future applications. The ability to tune chemical reactivity with microwave pulses holds great potential for enhancing the efficiency of chemical reactions across various fields, including materials science and pharmaceuticals. Imagine being able to spur a desired reaction while simultaneously minimizing unwanted byproducts—this could significantly streamline the synthesis of complex compounds.
Experimental Techniques and Findings
In their carefully controlled environment, Zhelyazkova and her collaborators made use of advanced equipment such as a time-of-flight mass spectrometer to analyze the products and verify reaction rates. Their experimental results revealed complexities in molecular interactions that were not fully explained by traditional theories. The researchers discovered that numerous magnetic sublevels of rotationally excited CO molecules contributed to the reactions, requiring a reevaluation of standard capture theory to account for these findings.
Conclusion
This revelation not only enhances our understanding of quantum effects in chemical reactions but could also have profound implications in the study of outer space chemistry, where similar conditions may apply. The researchers highlight that their method could be generalized across numerous molecular systems, paving the way for further investigations into molecular dynamics and chemical reactivity under designed conditions.
As this groundbreaking research continues to unfold, it could reshape our fundamental understanding of chemical processes, revealing the incredibly nuanced dance of molecules we thought we understood. With the power to suppress, enhance, and control reactions at the molecular level, the future of chemical synthesis may be revolutionized by simple microwave technology! Stay tuned for more developments in this thrilling area of scientific exploration.

 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 Česko (CS)
Česko (CS)
 대한민국 (KO)
대한민국 (KO)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)
 الإمارات العربية المتحدة (AR)
الإمارات العربية المتحدة (AR)