Revolutionary Probe Breaks Ground in Detecting Dangerous Hydrazine with Zero Background Fluorescence!
2025-04-28
Author: Ming
Unraveling the Dangers of Hydrazine: A Hidden Threat
Hydrazine (N2H4) is not just any chemical; it’s an extremely toxic organic amine with the alarming ability to ignite or explode upon contact with oxidants, air, or when exposed to high temperatures. Recognized as a Class B2 hazardous substance by multiple institutions—including the U.S. Environmental Protection Agency and the World Health Organization—it poses serious health risks that cannot be ignored. This underscores a pressing need for precise and reliable detection techniques.
A Game-Changing Detection Method!
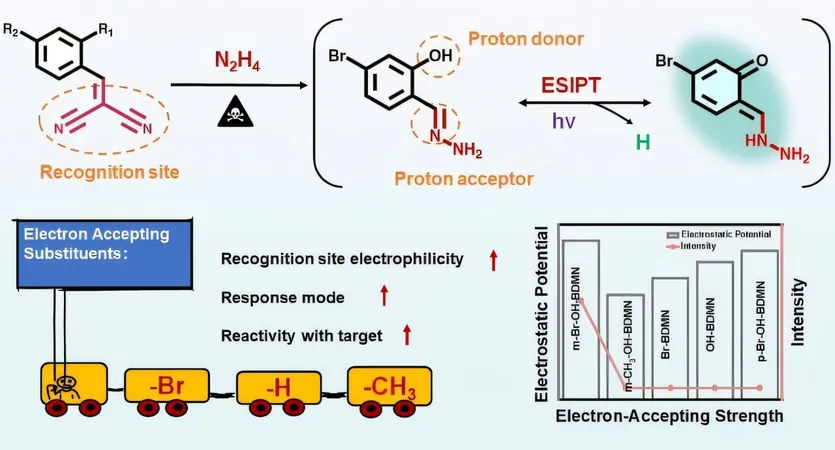
Enter the world of fluorescence turn-on probes. Utilizing a cutting-edge mechanism known as excited-state intramolecular proton transfer (ESIPT), these probes are known for their high sensitivity and minimal background interference, making them ideal for spotting hazardous substances. However, even these innovative designs face hurdles in specificity compared to similar compounds, leaving plenty of room for improvement. It's clear: fine-tuning these probes by tweaking their substituents poses a vital challenge.
Meet the Researchers Behind the Breakthrough!
A dedicated research team led by Prof. Dou Xincun from the Xinjiang Technical Institute of Physics and Chemistry of the Chinese Academy of Sciences has announced an exciting leap forward. They’ve launched a pioneering strategy for a zero-background fluorescence probe specifically tailored to enhance the detection of hydrazine with pinpoint accuracy.
How It Works: The Science Behind the Strategy
Their innovative approach, detailed in the journal Analytical Chemistry, involves fine-tuning the electron-accepting properties of the para-substituents in the probe design. By adjusting the positioning of the proton donor and the recognition site, they significantly boosted the probe's reactivity towards N2H4 and triggered the highly sensitive ESIPT process.
Crafting the Perfect Probes!
The researchers crafted a series of zero-background fluorescent probes, including m-Br-OH-BDMN, m-CH3-OH-BDMN, and several others, all employing dicyanoethylene as the recognition site. They manipulated the electron-accepting abilities of these probes by experimenting with various para-substituents and shifting the proton donor's position (either para or ortho relative to the recognition site).
Impressive Results: A Breakthrough in Detection!
The findings were striking: a probe showed dramatically improved reactivity with hydrazine when the hydroxyl group was positioned ortho to the recognition site, resulting in a captivating blue-green fluorescence emission. Notably, the m-Br-OH-BDMN probe, featuring bromine as its electron-acceptor, exhibited outstanding performance, boasting a low limit of detection at just 0.46 nM (14.72 ng/L), a rapid response time of only 1 second, and remarkable selectivity even amid 18 other potential interfering substances, including structural analogs of primary amines.
Why This Matters: Advancing Safety and Detection!
This groundbreaking advancement in hydrazine detection promises not just to enhance safety measures in various industries but also sets a new standard in the realm of hazardous substance detection. As researchers continue to refine these probes, the potential for saving lives and preventing disasters becomes increasingly feasible.


 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 Česko (CS)
Česko (CS)
 대한민국 (KO)
대한민국 (KO)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)
 الإمارات العربية المتحدة (AR)
الإمارات العربية المتحدة (AR)