Revolutionary Platinum-Nickel Catalyst Unleashed – A Game-Changer for Fuel Cell Efficiency!
2024-11-13
Author: Jia
Introduction
Electrocatalysis stands at the forefront of sustainable energy technology, making the development and optimization of catalysts more crucial than ever. Recently, a new breakthrough in platinum (Pt) and nickel (Ni) catalysts for oxygen reduction reactions (ORR) in fuel cells has captured the attention of scientists and industry experts alike.
The Challenge with Platinum
Historically, the use of platinum as a catalyst has been hampered by its inefficiency in facilitating the ORR due to its sluggish reactivity. However, Tohoku University's Di Zhang, a leading researcher in materials science, has revealed that altering the surface structure of platinum can significantly enhance its catalytic performance. "By tuning the surface structure, we can increase the reaction efficiency of platinum dramatically," Zhang states.
Introducing Surface Strain
At the heart of this innovation is a technique known as surface strain, where the atomic arrangement on the catalyst's surface is either compressed or expanded. This alteration facilitates improved reactivity, especially in the promising Pt-Ni system, which combines platinum and nickel to maximize performance.
Addressing Research Gaps
However, prior research has not fully delved into which specific regions of the catalyst are active during reactions or accounted for vital environmental factors such as pH levels that influence performance in real-world applications. To tackle these limitations, Zhang and an international research team developed a sophisticated new model that accurately mirrors real electrochemical conditions.
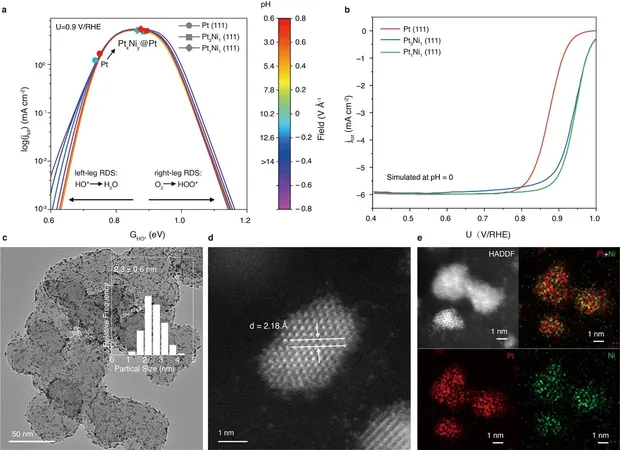
The Catalyst: Pt1Ni1@Pt/C
This led to the creation of a groundbreaking catalyst known as Pt1Ni1@Pt/C, characterized by its core-shell structure. The results have been nothing short of remarkable—a significant increase in activity over traditional platinum catalysts was achieved, demonstrating mass and specific activity measurements of 1.424 ± 0.019 A/mgPt and 1.554 ± 0.027 mA/cmPt², respectively.
Durability and Stability
Not only does this new catalyst exhibit exceptional activity, but it is also built to last. The research team reported that Pt1Ni1@Pt/C retains 98.4% of its catalytic performance even after an astounding 70,000 operational cycles. The innovative approach applied to anchor minuscule Pt-Ni nanoparticles (approximately 2.6 nm) to a carbon substrate ensures they remain stable, preventing any movement or clumping that could affect performance.
Optimizing Efficiency
The team further enhanced the catalyst's efficiency by creating a platinum-rich shell around the Pt-Ni core, applying a compressive strain that optimizes oxygen adsorption—a key factor in accelerating the reaction process.
Future Implications
With these advancements, researchers like Hao Li emphasize that the combination of new modeling techniques, cutting-edge material design, and advanced synthesis methods heralds a new era for platinum-nickel catalysts. "This research potentially paves the way for catalysts that are not only more efficient but also more durable, making them indispensable in the ongoing development of renewable energy technologies," Li concludes.
Conclusion
This innovative platinum-nickel catalyst could redefine the landscape of fuel cell technology and is poised to play a pivotal role in the transition toward greener energy solutions. As we stand on the brink of an energy revolution, these advancements paint a hopeful picture for a sustainable future. Don’t miss the opportunity to stay informed about this game-changing discovery!


 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)