Revolutionary Platinum-based Alloy Catalyst Boosts Methanol Fuel Cell Performance and Longevity!
2025-04-08
Author: Jia
Introduction
In the quest to combat environmental issues and mitigate the energy crisis, transitioning from traditional fossil fuels to clean, renewable energy sources has become paramount. Among innovative solutions, fuel cells stand out for their remarkable ability to convert chemical energy into electrical energy efficiently.
Methanol as a Promising Fuel
One of the most promising liquid fuels in this regard is methanol. Known for its high energy density, safety, and easy transport, methanol stands as a leading candidate for fuel cells. However, a primary hurdle in optimizing methanol oxidation is the rapid deactivation of catalysts due to poisoning from byproducts of the reaction, particularly carbon monoxide. These intoxicating species can cling to catalysts, obstructing their functionality.
The Need for Improved Catalysts
Thus, improving catalyst activity and resistance to these poisons remains essential for advancing direct methanol fuel cells (DMFCs).
Breakthrough in Catalyst Development
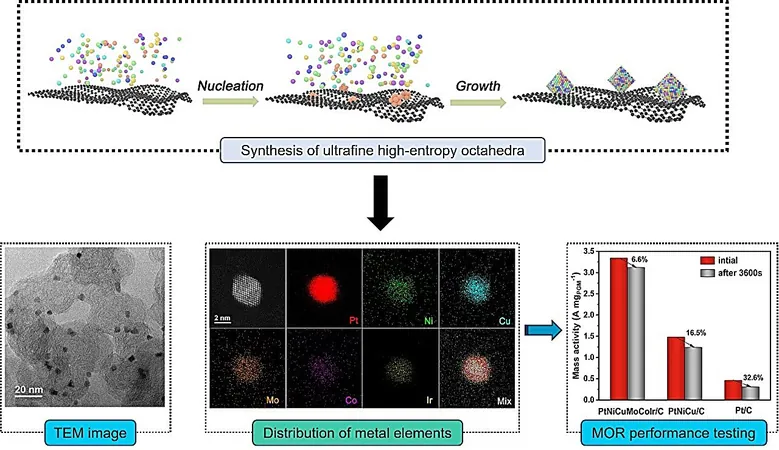
Addressing this pressing issue, a pioneering research team led by Prof. Zhang Tierui at the Technical Institute of Physics and Chemistry of the Chinese Academy of Sciences (CAS) has unveiled a cutting-edge catalyst that drastically enhances both the efficiency and longevity of methanol oxidation reactions. This breakthrough involves the development of ultrafine platinum-based high-entropy alloy (HEA) octahedra, signaling a remarkable leap forward in the performance of DMFCs.
Significant Findings
This groundbreaking research, published in the esteemed journal Matter on April 8, outlines significant findings related to the catalysts' design and application. The platinum-based octahedral materials feature exposed crystal facets that typically exhibit excellent activity in various electrocatalytic processes. Nevertheless, their high surface energy can lead to instability and aggregation, resulting in inefficient use of the catalyst's active sites.
Engineering Ultrafine HEA Octahedra
In a remarkable approach, Prof. Zhang’s team engineered ultrafine HEA octahedra composed of a blend of multiple metal elements. By integrating various elements into the structure, they successfully lowered the surface energy of the platinum-based nano-octahedra, resulting in stable configurations with edge lengths well below 3 nanometers—much smaller than previous iterations.
Experimental Results
Experimental results demonstrate that as the diversity of metal elements increases in the alloy, the size of the octahedra diminishes correspondingly. Notably, the average edge length of a unique senary alloy, which consists of six distinct metal elements, measures merely 2.8 nanometers.
Enhanced Performance in Methanol Oxidation
In a series of electrochemical tests accompanied by theoretical calculations, it was confirmed that the synergistic effects of the metal elements significantly enhance the electronic structure of platinum (Pt). The findings reveal that in methanol oxidation reactions, the senary alloy exhibited superior performance in both activity and resistance to poisoning, outperforming both ternary alloys (composed of three metal elements) and commercial platinum-on-carbon (Pt/C) catalysts.
Implications for Renewable Energy
This innovative catalyst development not only promises to advance the efficiency of methanol fuel cells but could also have profound implications for the broader field of renewable energy technologies. As the world pushes for sustainable energy solutions, this research unveils a potential pathway to a cleaner energy future, proving that with the right scientific advancements, the dream of efficient, renewable energy sources may soon become a reality.
Conclusion
Stay tuned as we continue to track advancements in clean energy technology that could change the course of our planet’s future!


 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 Česko (CS)
Česko (CS)
 대한민국 (KO)
대한민국 (KO)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)
 الإمارات العربية المتحدة (AR)
الإمارات العربية المتحدة (AR)