Revolutionary Optogenetic Tool Transforms Protein and RNA Control in Living Cells
2025-07-28
Author: Mei
Breakthrough in Living Cell Manipulation
A pioneering research team at KAIST, led by Professor Won Do Heo from the Department of Biological Sciences, has unveiled a groundbreaking optogenetic platform called RELISR (REversible LIght-induced Store and Release). This innovative tool allows for unprecedented precision in controlling the storage and release of proteins and mRNAs within living cells and animal tissues.
Exceeding Limitations of Traditional Systems
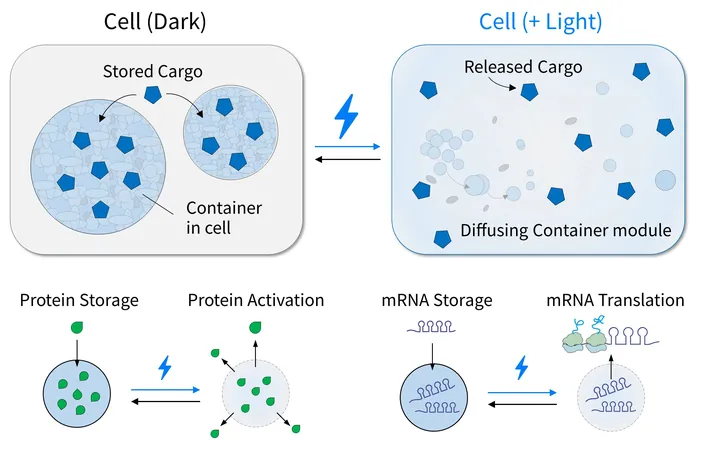
Traditional optogenetic systems have faced challenges due to their dependence on non-specific interactions that can inadvertently trap or release unwanted cellular molecules. RELISR marks a significant advancement by utilizing highly specific interactions—between proteins (nanobody-antigen) and proteins-RNA (MCP-MS2)—to achieve selective, reversible compartmentalization of target molecules in engineered, membrane-less condensates.
Light-Induced Precision Control
Under dark conditions, RELISR effectively sequesters target proteins and mRNAs, isolating them from the cellular environment. When stimulated by blue light, these condensates swiftly dissolve, liberating the molecules to regain their functionality. This mechanism facilitates rapid and reversible modulation of molecular activities in response to light, providing scientists with a powerful tool for experimental manipulation.
Versatile Applications Across Cell Types
The research team showcased RELISR’s capacity to regulate protein activity and mRNA translation across various cell types, including cultured neurons and mouse liver tissue. The findings reveal that RELISR offers more robust and reversible control compared to previous optogenetic frameworks, which typically focused on spatial translocation.
Setting New Standards in Optogenetics
Prior systems like LARIAT were restricted mainly to trapping proteins or mRNAs in response to light, which limited their functional versatility. In contrast, RELISR opens new horizons by not only facilitating the targeted storage of these molecules but also allowing their swift release when needed. This capability empowers researchers to both confine and restore molecular functions with pinpoint timing.
A Vision for the Future of Molecular Medicine
Professor Heo emphasized the vast potential of RELISR: "This versatile optogenetic tool enables precise control over protein and mRNA function at specific times and locations in living systems. We foresee it being broadly applicable in studying cell signaling, neural circuits, and therapeutic innovation. Moreover, integrating RELISR with genome editing or tissue-targeted delivery could significantly enhance its applicability in molecular medicine."
Collaborative Scientific Effort
This groundbreaking research, led by first author Dr. Chaeyeon Lee, was conducted under the mentorship of Professor Heo, with valuable contributions from Dr. Daseuli Yu and Professor YongKeun Park from the Department of Physics, who provided essential quantitative imaging analyses of the biophysical changes caused by RELISR in living cells.


 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 Česko (CS)
Česko (CS)
 대한민국 (KO)
대한민국 (KO)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)
 الإمارات العربية المتحدة (AR)
الإمارات العربية المتحدة (AR)