Revolutionary One-Step Test for Monkeypox Detection: Fast, Simple, and Specific!
2025-05-20
Author: Rajesh
The alarming rise of Monkeypox virus (MPXV) infections has taken the world by storm, demanding immediate attention for public health safety. As cases surge, the need for a quick, precise, and user-friendly diagnostic method has become more critical than ever to tackle outbreaks effectively.
Breakthrough Assay Combines LAMP and CRISPR Technology!
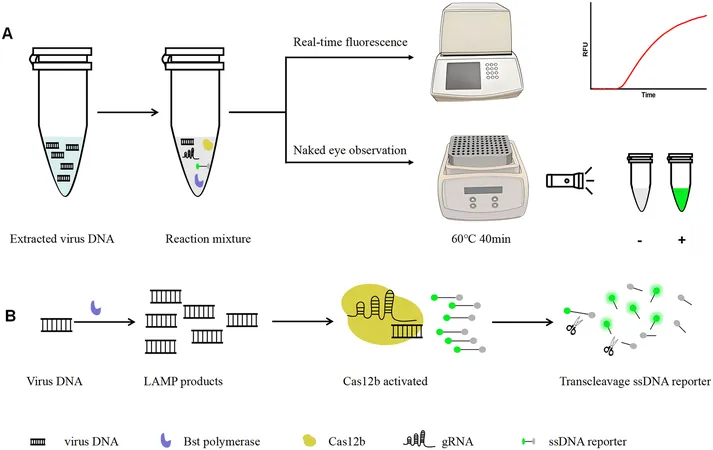
In a groundbreaking study, scientists have introduced an innovative one-step assay that melds loop-mediated isothermal amplification (LAMP) with CRISPR/Cas12b technology—revolutionizing the way we detect MPXV. This streamlined process takes under 40 minutes and requires no opening of the reaction tube, surpassing traditional methods like real-time quantitative PCR (qPCR) in speed. The results can easily be interpreted either via real-time fluorescence or visible with the naked eye!
Unmatched Sensitivity and Specificity!
This remarkable assay boasts a limit of detection (LOD) of 6.5 copies per reaction, ensuring high accuracy without cross-reactivity to other common pathogens such as HSV or EBV. Clinical evaluations show this test achieving an outstanding 100% sensitivity and specificity when benchmarked against qPCR. Out of 113 clinical samples, every positive case was detected, paving the way for reliable diagnostics.
Ideal for Resource-Limited Settings!
What sets this method apart is its accessibility. Only a portable heat block or water bath, along with a blue light or ultraviolet flashlight, is required for visual detection, making it perfect for deployment in areas with limited resources. This one-pot solution is particularly promising for point-of-care testing, enabling rapid diagnosis anywhere.
A Leap in Public Health Surveillance!
Monkeypox, historically confined to West and Central Africa, has now spread globally with over 124,000 confirmed cases reported by the WHO. Quick identification of this viral threat is critical to controlling its spread. Conventional diagnostic methods can be slow and complex, requiring specialized personnel and equipment. But with this new approach, the future looks brighter for public health!
What This Means for the Future!
Leveraging the combined power of LAMP and CRISPR, this cutting-edge assay could dramatically enhance the world’s capacity to combat infectious diseases proactively. The implications for rapid testing in various settings—ranging from airports to remote villages—are profound, potentially reducing transmission rates of monkeypox and other infectious threats. As we face increasing health challenges, innovations like this remind us that the future of diagnostics is here.
Stay tuned as this exciting technology unfolds, paving the way for a healthier, safer world!


 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 Česko (CS)
Česko (CS)
 대한민국 (KO)
대한민국 (KO)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)
 الإمارات العربية المتحدة (AR)
الإمارات العربية المتحدة (AR)