Revolutionary One-Step Process Enhances CO₂ Regeneration Efficiency for Carbon Capture!
2024-11-08
Author: Sarah
Introduction
As the world grapples with the dire consequences of climate change, the quest to develop sustainable energy sources continues to grow. While renewable energy solutions hurry to replace fossil fuels, a critical interim solution lies in carbon capture technologies that target carbon dioxide (CO₂) emissions. But with conventional systems often proving too costly or energy-heavy, researchers are racing against time to find innovative alternatives.
Groundbreaking Study from Rice University
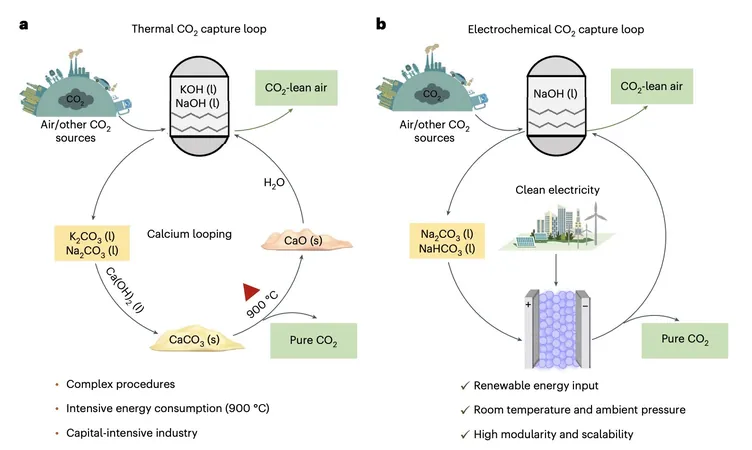
A groundbreaking study from Rice University introduces a game-changing one-step electrochemical regeneration process that promises to significantly enhance the efficiency of CO₂ capture from carbon-containing solutions. Their findings, recently published in *Nature Energy*, could usher in a new era of energy-efficient carbon capture at industrial facilities and help mitigate air pollution globally.
Simplifying the Regeneration Process
Traditionally, carbon capture and regeneration methods have been energy-intensive and complicated, requiring substantial heat and multiple steps. However, researchers Xiao Zhang, Zhiwei Fang, and their team have unveiled a modular porous solid electrolyte (PSE) reactor that simplifies the process considerably.
The Revolutionary Method
In a stunning demonstration, the researchers reported that bicarbonate solutions can be continuously transformed back into pure CO₂ gas through a combination of hydrogen evolution reaction and hydrogen oxidation reaction (HER/HOR) redox electrolysis. "Our PSE reactor selectively splits sodium bicarbonate or carbonate solutions—commonly found post-CO₂ absorption—into sodium hydroxide and high-purity CO₂," explained the researchers. Astonishingly, this method consumes no additional chemicals and produces zero by-products.
Impressive Results and Durability
Initial testing on the modular solid electrolyte reactor revealed impressive results: a Na⁺-ion transport number reaching approximately 90%, a retention of capture capacity also around 90%, and very low energy requirements (50 kJ/mol at 1 mA/cm² and 118 kJ/mol at 100 mA/cm²). Moreover, the reactor's durability shines through, having maintained stability for over 100 hours of operation.
Potential for Industrial Application
What truly excites researchers is the rapid carbon regeneration rates achieved—up to 1 A/cm² (approximately 18 mmol/cm²/h), showcasing its potential application in industrial scenarios. "These significant outcomes underscore the revolutionary potential of our proposed method for CO₂ regeneration, suggesting a more efficient alternative to conventional thermal processes," pointed out Zhang and Fang.
Future Plans and Conclusion
As the team looks ahead, they plan to scale up their reactor design, striving for even greater efficiency in carbon capture technologies. This pioneering study not only paves the way for a promising alternative method of CO₂ regeneration but might also inspire further research into new carbon capture solutions that could play a pivotal role in addressing the climate crisis.
With this exciting advancement, the horizon for carbon capture technology looks brighter than ever. Stay tuned as we follow the developments in this revolutionary field, which could ultimately help safeguard the planet from the severe effects of carbon emissions.


 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 Česko (CS)
Česko (CS)
 대한민국 (KO)
대한민국 (KO)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)
 الإمارات العربية المتحدة (AR)
الإمارات العربية المتحدة (AR)