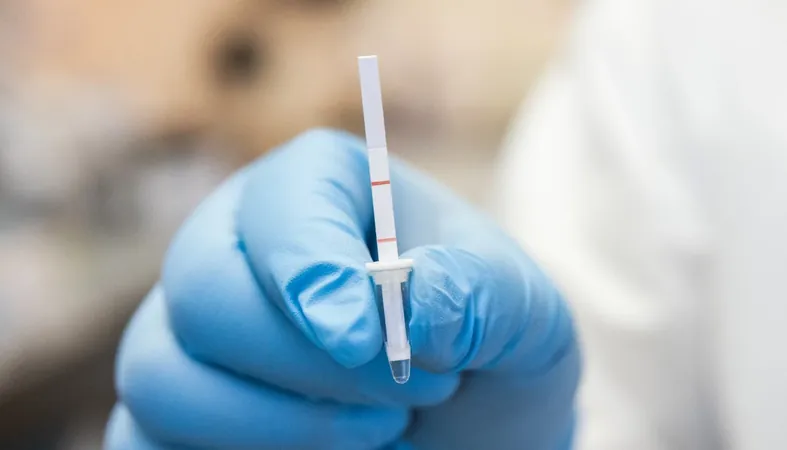
Revolutionary ‘One-Pot’ Test Diagnoses TB in Just One Hour!
2025-09-23
Author: Arjun
A Game-Changer in Tuberculosis Diagnosis!
For those grappling with tuberculosis (TB), one of the world’s deadliest infections, timely diagnosis has always been a significant hurdle. Enter a groundbreaking new solution: a ‘one-pot’ test leveraging the Nobel Prize-winning CRISPR gene-editing technology to provide TB diagnoses in less than an hour!
Breaking Down Barriers to TB Detection
In 2023, nearly 11 million people globally were infected with TB, with two-thirds concentrated in just eight countries across Asia and Africa. Alarmingly, a staggering one-third of new cases remain undiagnosed each year, largely due to access issues and the complexity of traditional testing methods that typically involve sputum samples, which can be challenging to obtain.
Meet ActCRISPR-TB: The Future of TB Testing
The innovative study introduces the ActCRISPR-TB test, an advanced method that allows diagnosis from not only saliva but also stool and spinal fluid samples. This improvement dramatically simplifies the process, making it easier for healthcare providers to effectively screen for TB.
How It Works: Harnessing CRISPR Technology
The ActCRISPR-TB test employs a CRISPR-based assay that amplifies and detects DNA signals from Mycobacterium tuberculosis, the bacteria responsible for TB. Zhen Huang from Tulane University, the lead researcher, explained how the test has evolved from a two-step system to a more user-friendly single-step procedure suitable for clinical situations.
User-Friendly and Fast Results
What’s even more impressive? The test is so simple that it can be self-administered. Just mix a sample with the reagents in a tube, incubate, and results show up in about an hour. If the bacterial load is high, it takes as little as 15 minutes to get a positive result, making it incredibly efficient—even for asymptomatic patients who could unknowingly spread the infection.
The Promise of On-Site Testing
Current testing methods often revolve around sputum samples, which are not easily attainable for everyone, especially vulnerable populations like HIV patients and children. Traditional cultures can take weeks for results. With ActCRISPR-TB, there's hope for immediate on-site testing in communities, particularly in remote areas where medical resources are scarce.
Expert Opinions: A Big Leap Forward
Bhushan Toley, a researcher at the Indian Institute of Science, describes ActCRISPR-TB as a remarkable advancement in TB diagnostics, highlighting its minimal instrumentation and exceptional sensitivity from tongue swabs. Yet, he notes the need for further simplification to maximize its usability in community settings.
Looking Ahead: A Bright Future for TB Screening
While progress has been made, Huang acknowledges that there's still work to do. The team is focused on validating their findings in larger, more diverse populations and optimizing the testing protocol to ensure that ActCRISPR-TB can ultimately serve as a reliable solution for widespread TB screening.


 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 Česko (CS)
Česko (CS)
 대한민국 (KO)
대한민국 (KO)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)
 الإمارات العربية المتحدة (AR)
الإمارات العربية المتحدة (AR)