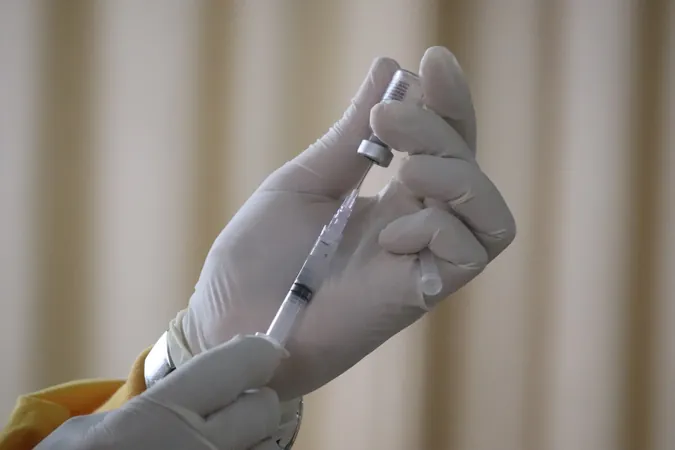
Revolutionary NIH Grant Sparks Hope for a Childhood HIV Vaccine
2025-09-20
Author: Rajesh
A Groundbreaking Step in HIV Prevention
A multi-institutional team led by Weill Cornell Medicine has secured a staggering $20.8 million grant from the National Institute of Allergy and Infectious Diseases, part of the esteemed National Institutes of Health. This grant is aimed at advancing the preclinical development of an experimental HIV vaccine that could change the course of public health.
The Urgent Need for an HIV Vaccine
With approximately 1.3 million new HIV infections recorded in 2024, according to the World Health Organization, the demand for innovative solutions has never been more pressing. Currently, around 41 million people are living with HIV, with treatment regimens that last a lifetime. A successful vaccine could drastically reduce these numbers and prevent future infections.
The Promise of Early Childhood Immunization
Early studies indicate that this new vaccine is safe and may yield effective results when administered during infancy. The grant will allow researchers to optimize the vaccine for clinical trials in regions heavily affected by HIV, focusing on the potential for childhood inoculation.
Dr. Sallie Permar, the lead investigator and chair of Pediatrics at Weill Cornell Medicine, envisions a future where inducing strong immunity against diverse HIV variants in children could be the key to ending the decades-long HIV pandemic.
Cracking the HIV Code: The Scientific Challenge
Since the virus was identified as the cause of AIDS in 1983, creating an effective vaccine has posed significant challenges. The HIV virus rapidly mutates, leading the immune system on a perpetual hunt for effective responses. While rare antibodies do exist that can neutralize various HIV strains, generating a vaccine that triggers an adequate immune response has remained elusive.
Innovative Solutions on the Horizon
Current advancements revolve around engineered versions of HIV’s outer-envelope protein complex, Env. These specially designed proteins maintain their integrity while prompting the immune system to produce broadly neutralizing antibodies (bnAbs). The experimental vaccine to be tested utilizes the latest optimized trimer structure, the BG505 GT1.1 SOSIP trimer, aimed at guiding antibody responses effectively over time.
Pioneering Pediatric Trials
Traditionally, vaccines are tested on children post-approval in adults. However, the preliminary trials suggest that the vaccine's effectiveness in stimulating an immune response is greater in younger immune systems. This insight positions the vaccine for a unique opportunity to be included in the childhood immunization schedule.
Dr. Permar highlights that the real chance for an effective vaccine could lie in its application during childhood.
Optimizing the Vaccine Experience
The key objective of this new research program is to refine the Env trimer vaccine and pave the way for impending pediatric clinical trials. Researchers will optimize the dosage, the immune-boosting adjuvants, and the vaccination schedule to ensure maximum efficacy.
Considering Interactions with Other Vaccines
Dr. Genevieve Fouda will lead a sub-project examining how existing childhood vaccines might influence the effectiveness of the experimental HIV vaccine. Particularly in sub-Saharan Africa, where vertical transmission rates are high, newborns could benefit from simultaneous administration of anti-HIV bnAbs during breastfeeding alongside their standard immunizations.
This ambitious initiative could not only protect the most vulnerable populations but also revolutionize the fight against HIV, heralding a new era in vaccine development.

 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 Česko (CS)
Česko (CS)
 대한민국 (KO)
대한민국 (KO)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)
 الإمارات العربية المتحدة (AR)
الإمارات العربية المتحدة (AR)