Revolutionary New Tool Unlocks Secrets of Gene Mutations Within Living Human Cells!
2024-11-14
Author: Wei
Introduction
In the realm of biomedical research, gene mutations play a double-edged sword—some confer resistance to diseases like diabetes, while others may predispose individuals to severe conditions such as cancer. Understanding these mutations is crucial; however, modifying genetic material inside human cells presents a formidable challenge due to the complexity embedded in our 3 billion base pairs of DNA.
Introduction of HACE
Enter a groundbreaking innovation from a team at Harvard, where researchers have developed a state-of-the-art tool called Helicase-Assisted Continuous Editing (HACE). This new method allows scientists to implement mutations in specific genes of interest without jeopardizing the integrity of the entire genome. In a recently published article in Science, the potential of HACE has been highlighted, signifying a monumental leap in genetic research.
Comments from Researchers
First author Xi Dawn Chen, a dedicated researcher in synthetic biology, expressed the excitement surrounding this tool: "The development of HACE enhances our ability to manipulate evolution within human cells directly," she stated. The targeted mutagenesis enabled by this method sets it apart from existing techniques, which often entail indiscriminate alterations across numerous genes.
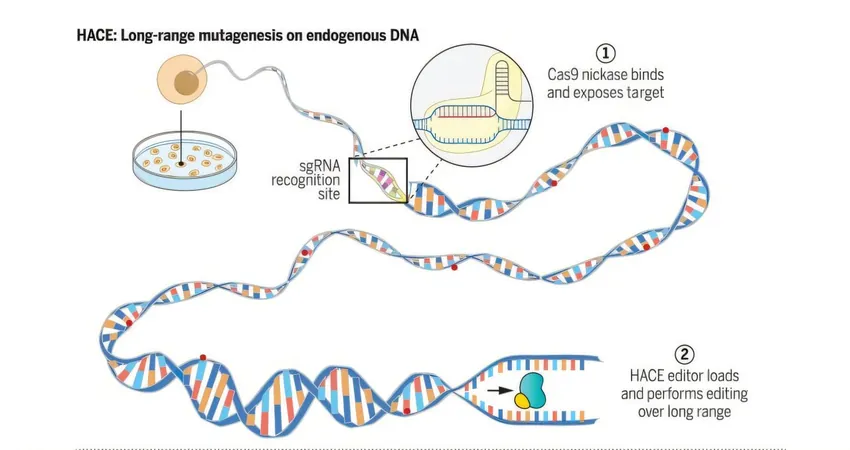
How HACE Works
HACE integrates the capabilities of a helicase—an enzyme that effectively "unzips" DNA—with the cutting-edge CRISPR-Cas9 gene-editing technology. By directing this powerful duo to specific gene locations, researchers can introduce precise mutations in targeted regions, akin to finding a specific house in a vast neighborhood rather than wandering aimlessly.
Demonstration of Efficacy
In a compelling demonstration of HACE's efficacy, researchers identified mutations conferring drug resistance in the MEK1 gene, often a target in cancer therapies that frequently fall short due to adaptive mutations in tumor cells. By utilizing HACE, the team was able to sequence these mutations, revealing critical insights into the nature of drug resistance mechanisms that impede effective treatment.
Research Extending to Other Genes
Furthermore, the research extended to SF3B1, a gene associated with RNA splicing, commonly altered in various blood cancers. By leveraging HACE, the scientists could pinpoint the mutations responsible for disrupting RNA assembly, a significant breakthrough in understanding cancer pathogenesis.
Collaboration and Broader Implications
Collaboration with prominent institutions like Harvard Medical School and Dana-Farber Cancer Institute provided additional support in exploring how DNA regulatory region changes influence protein production in immune cells, which are increasingly seen as targets for innovative cancer immunotherapies.
Future Perspectives
Looking towards the future, renowned researcher Bradley Bernstein posits that tools like HACE hold the potential for sweeping alterations to gene regulatory sequences. Such advancements could synergize with artificial intelligence, opening new therapeutic avenues to 'correct' gene activity and significantly improve patient outcomes in genetic diseases.
Conclusion
As this revolutionary tool continues to evolve, the future of precision medicine shines brighter than ever, promising a new era in our battle against genetically linked diseases. Stay tuned to witness the unfolding of groundbreaking treatments that were once deemed out of reach!


 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)