Revolutionary New Polymers: Dense Cyclic Units Unveiled in Carbon-Carbon Main Chain Design
2025-08-25
Author: Rajesh
Unlocking the Secrets of Carbon-Carbon Main Chain Polymers
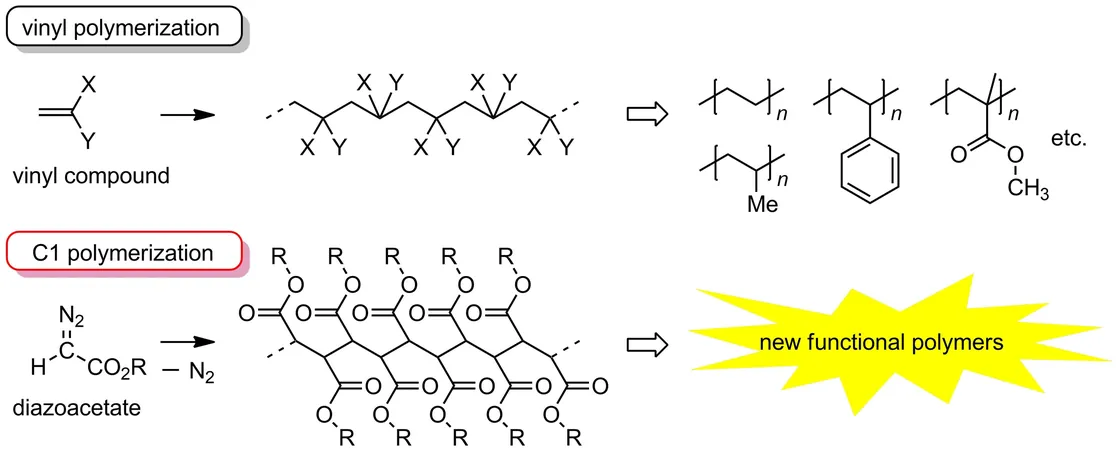
Polymers with a carbon-carbon backbone are essential in the manufacturing of everyday plastics, with vinyl polymerization being the most common method. This process uses vinyl compounds, leveraging the reactivity of double bonds to form two-carbon unit chains, yielding materials like polyethylene and polypropylene—ubiquitous in countless applications.
A Game-Changer: C1 Polymerization
But there's a new player in town: C1 polymerization. Unlike its vinyl counterpart, this innovative method constructs carbon chains from one-carbon units, tapping into the unique properties of monomers like diazoacetate and sulfoxonium methylide. This technique allows the introduction of functional groups at each carbon, potentially leading to unique functionalities and enhanced properties that traditional vinyl polymers simply can't match.
Breakthrough from Ehime University
A trailblazing team from Ehime University has made significant strides in this field by developing "C1 cyclopolymers." Through a process known as cyclopolymerization, they create structures where every carbon in the main chain forms part of a cycle, resulting in densely packed cyclic units that outperform traditional cyclopolymers formed from divinyl compounds.
Overcoming Limitations in Synthesis
Historically, the use of bifunctional diazoacetates posed synthetic challenges, restricting the types of polymers that could be created. However, in a groundbreaking study published in Macromolecules, the Ehime team unveiled a new method using pentaerythritol—a polyhydric alcohol—as a starting point to fabricate these essential diazoacetates.
Innovative Ring Structures and Enhanced Properties
Their efforts not only led to the synthesis of new monomers but also opened the door to creating C1 cyclopolymers with a breathtaking variety of ring sizes ranging from 9 to 19 members, along with functional groups such as urethane linkages that can form hydrogen bonds, enriching the cyclic framework.
Higher Temperatures, Better Materials
In testing these innovative polymers, the team discovered that their glass transition temperatures were significantly elevated compared to C1 polymers lacking cyclic structures. This is a promising development for applications requiring durability and resilience.
Paving the Way for Future Innovations
These pioneering findings not only expand our understanding of carbon-carbon main chain polymers but also pave the way for new materials with tailored properties, potentially revolutionizing the polymer industry as we know it.


 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 Česko (CS)
Česko (CS)
 대한민국 (KO)
대한민국 (KO)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)
 الإمارات العربية المتحدة (AR)
الإمارات العربية المتحدة (AR)