Revolutionary 'Nanosnag' Detection Method Set to Transform Vaccine Production Quality Checks!
2025-03-12
Author: Mei
Introduction
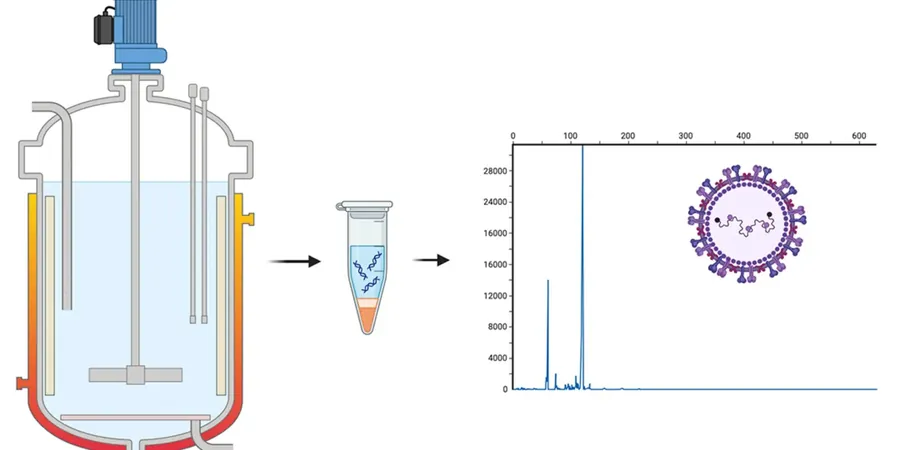
In a groundbreaking advance for the pharmaceutical industry, researchers at Carnegie Mellon University have unveiled a revolutionary virus detection technique that could drastically enhance the quality control measures in vaccine manufacturing. As the demand for viral vaccines escalates to address global health crises, the ability to efficiently quantify viral genomes is becoming increasingly crucial.
The Nanosnag Technique
The team from the Schneider Lab has introduced an innovative method that utilizes a concept they refer to as a 'nanosnag'—a short piece of double-stranded DNA attached to viral genomes. This nanosnag effectively alters the mobility of the viral genome, allowing it to move through a gel-like matrix more slowly in the presence of an electric field.
Efficiency and Speed
Traditional methods like gel electrophoresis and polymerase chain reaction (PCR) can be time-consuming and less efficient. However, Schneider's technique promises a swift 10-minute analysis, leveraging surfactants to create a gel-like environment that contrasts with the more complex polymer structures used in older methods.
Technical Insights
Interestingly, the researchers discovered that attaching a mere 30-base fragment of DNA to a larger 5,000-base viral genome significantly impacts its migration through the matrix. This unexpected outcome sheds light on the polymer physics at play, showcasing how the stiffness of the nanosnag alters the travel path of the viral genome.
Specificity and Applications
One of the standout features of this technology is its specificity. The nanosnag fragment selectively binds to particular sequences of the viral genome, allowing researchers to precisely isolate the viral components from other nucleic acids in the sample.
Collaboration and Future Prospects
In a significant step forward, Schneider is collaborating with pharmaceutical companies to integrate this technology into manufacturing processes. One of the challenges faced is the presence of cell debris and other contaminants that can muddle detection.
Conclusion
The implications of this innovation are enormous—streamlining vaccine production, enhancing quality control, and ultimately improving global health outcomes in the fight against diseases.
 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 Česko (CS)
Česko (CS)
 대한민국 (KO)
대한민국 (KO)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)
 الإمارات العربية المتحدة (AR)
الإمارات العربية المتحدة (AR)