Revolutionary Nanoislands Supercharge Platinum Catalysts: A Breakthrough in Chemical Reactions!
2025-01-29
Author: Daniel
Introduction
Noble metals, prominently platinum, are well-recognized as powerful catalysts that can significantly accelerate chemical reactions, especially in processes like hydrogenation (the method of adding hydrogen atoms to a molecule). Researchers at the University of California, Davis, led by the innovative Professor Bruce Gates from the Chemical Engineering Department, are on a groundbreaking mission to develop platinum catalysts that are not only highly efficient but also remarkably stable during chemical reactions.
Research Presentation
Their pioneering research has been featured in the prestigious journal, Nature Chemical Engineering, alongside a detailed research brief, offering insights into their findings.
Previous Studies
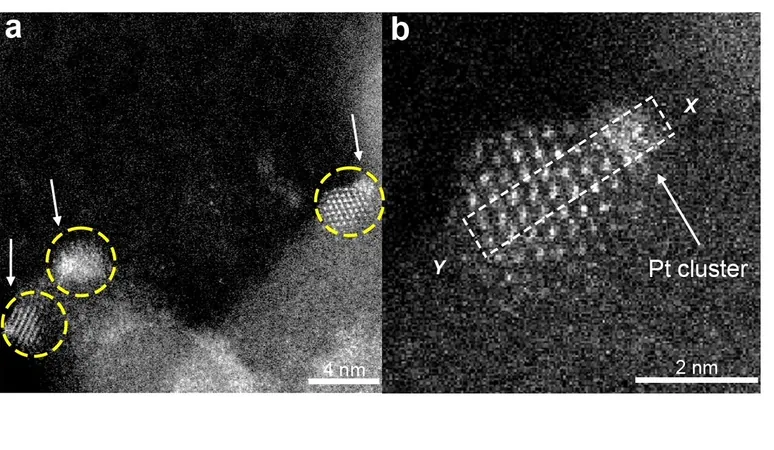
Historically, previous studies have indicated that platinum configured in minuscule clusters of just a few atoms outperforms individual platinum atoms or larger nanoparticles when it comes to hydrogenation processes. However, a major challenge has been that these tiny clusters tend to agglomerate into larger particles, thereby diminishing their efficiency.
The Game-Changing Concept
Enter Yizhen Chen, a postdoctoral scholar within the Gates Catalysis Research Group, who built upon a game-changing concept introduced by Jingyue Liu, now at Arizona State University. This concept involves 'trapping' platinum clusters on incredibly small islands made from cerium oxide, supported on a clean silica surface. Each of these nanoislands operates like its own tiny chemical reactor.
Successful Demonstration
The collaborative team, comprising Chen, Gates, and their colleagues, successfully demonstrated the production of these platinum clusters. Remarkably, they exhibited impressive catalytic activity in the hydrogenation of ethylene—a vital reaction in many industrial applications. Even more importantly, these clusters maintained their stability, withstanding the harsh conditions often encountered in chemical reactions.
Impact on the Chemical Industry
This breakthrough in creating confined metal clusters marks a significant advancement for the chemical industry, potentially paving the way for the development of stable and efficient catalysts. As the demand for sustainable and efficient chemical processes grows, this innovative approach could play a crucial role in reducing waste and improving the effectiveness of catalyst applications, transforming how we think about chemical reactions in both industrial and laboratory settings.
Conclusion
Stay tuned as this fascinating research evolves, heralding a new dawn for catalyst technology!

 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 Česko (CS)
Česko (CS)
 대한민국 (KO)
대한민국 (KO)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)