Revolutionary Nanobody Scaffolds Unlock Secrets of the Tiniest Proteins
2025-08-21
Author: Mei
Breakthrough in Cryo-EM Technology
A team of innovative scientists from the Rosalind Franklin Institute, the University of Oxford, and Diamond Light Source has achieved a groundbreaking milestone in protein imaging. Their new technique now enables the imaging of minuscule proteins using cryo-electron microscopy (cryo-EM), with findings detailed in the latest edition of Nature Chemical Biology.
Introducing the Tiny Powerhouses
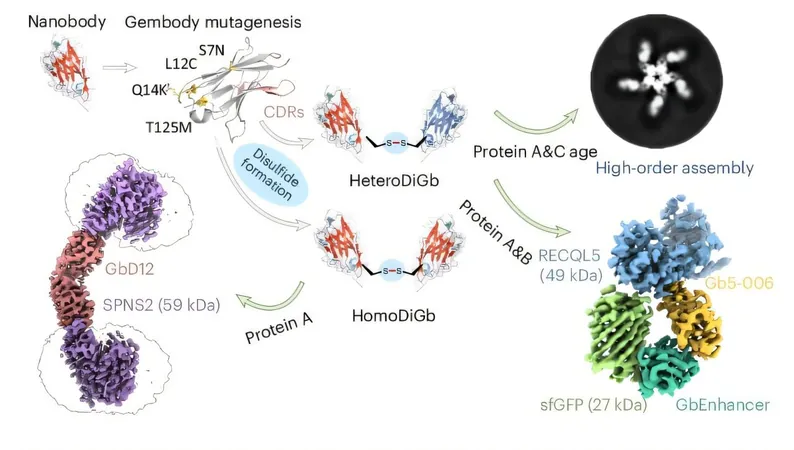
Through the creation of advanced bifunctional, bispecific nanobody scaffolds, researchers have tackled one of the most significant hurdles in structural biology: visualizing proteins smaller than 50 kDa. Remarkably, they successfully resolved the structure of hen egg white lysozyme, measuring in at just 14 kDa—making it the tiniest protein ever imaged by cryo-EM!
This revolutionary advancement is critical, as approximately 75% of human protein-coding genes yield proteins within this small size category. Many of these proteins are vital to cellular function and are integral to understanding various health conditions.
Unraveling the Challenges of Small Proteins
In recent years, single-particle cryo-EM has emerged as a leading method for determining protein structures. Among its many advantages is the ability to visualize molecular details in a nearly native state. However, capturing small proteins has remained an uphill battle due to their low signal-to-noise ratio, posing challenges in data processing and resulting in historically low-resolution images.
Innovative Solutions Through Nanobody Scaffolds
The newly developed nanobody scaffolds address these challenges by attaching small proteins to the ends of bifunctional, bispecific nanobodies, effectively increasing their apparent size and enhancing their visual clarity. This innovative approach opens up a world of possibilities for structural research and beyond, according to co-author Gangshun Yi.
"This has been the biggest challenge I've ever faced, but it has also been incredibly rewarding as I advance my career as a researcher. This technique showcases immense potential for widespread application across various scientific domains due to its versatile bispecific capabilities," Yi states.
A Lab Experiment Turned Major Discovery
The genesis of this research was as organic as it was serendipitous. Dimitrios Mamalis, a joint Ph.D. student at the University of Oxford and the Franklin Institute, recounted, "It began as a casual experiment on a Friday afternoon, not initially central to our main research focus, yet it turned into an outstanding outcome."
Mingda Ye's initial work with gembodies for crystallography initiated the inquiry into whether a similar methodology could be applied to cryo-EM, leading to this remarkable discovery.
Modular and Efficient: A Game Changer for Protein Study
Unlike other methods, this approach is modular, eliminating the tedious re-optimization process for different proteins. It even allows for the simultaneous study of two proteins, irrespective of their sizes, by mounting them on opposing ends of the scaffold.
Collaboration Breeds Innovation
Professor Ben Davis, science director at the Franklin and co-author of the study, emphasized the power of teamwork in this achievement. "This collaborative effort blossomed from similarities between crystallization and covalent protein trapping techniques. The sidechain-to-sidechain conjugation has shown remarkable efficiency. This method offers an exciting, pragmatic approach to protein study."




 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 Česko (CS)
Česko (CS)
 대한민국 (KO)
대한민국 (KO)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)
 الإمارات العربية المتحدة (AR)
الإمارات العربية المتحدة (AR)