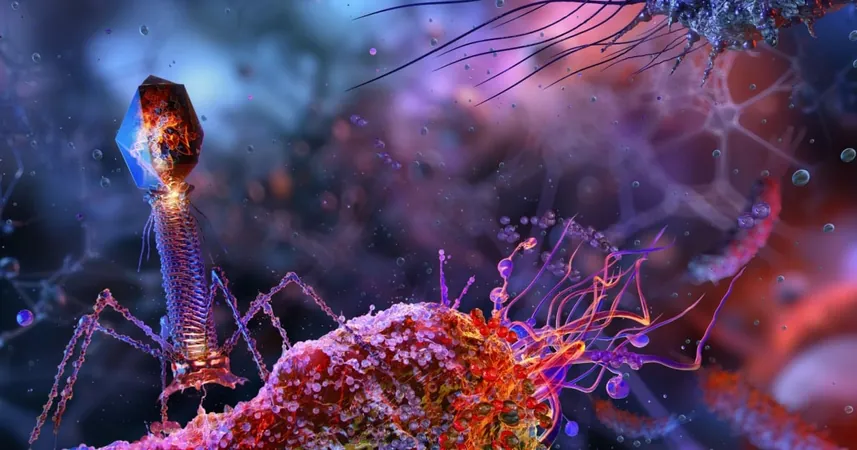
Revolutionary Move: UK Regulator Backs Bacteriophage Breakthroughs Against Superbugs!
2025-06-04
Author: Siti
UK Takes Bold Step in Antimicrobial Battle
In an unprecedented move, the UK’s Medicines and Healthcare products Regulatory Agency (MHRA) is stepping up the fight against antimicrobial resistance by embracing bacteriophage technology! This innovative approach aims to harness the power of viruses that specifically target and kill bacteria, offering new hope in the ongoing battle against stubborn infections.
Guidance to Transform Research into Reality
The MHRA has unveiled its first official guidance on bacteriophages, designed to help researchers navigate the complex regulatory landscape. This crucial framework outlines the quality and safety standards necessary for companies eager to develop bacteriophage therapies, bringing them one step closer to market.
A Game-Changer in Infection Treatment?
As traditional antibiotics face dwindling effectiveness, the potential of bacteriophages shines brighter than ever. By providing precise treatments that can target specific bacterial strains, these viral agents could revolutionize how we approach infections, ultimately saving countless lives.
Join the Fight Against Superbugs!
The MHRA's backing signals a significant shift in medical science, centering around the urgent need to combat the growing threat of antimicrobial resistance. Researchers and pharmaceutical innovators are now encouraged to explore this promising avenue, potentially paving the way for groundbreaking treatments that could change the landscape of healthcare.




 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 Česko (CS)
Česko (CS)
 대한민국 (KO)
대한민국 (KO)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)
 الإمارات العربية المتحدة (AR)
الإمارات العربية المتحدة (AR)