Revolutionary Monthly HIV Prevention Pill Unveiled by Merck & Co.
2025-09-11
Author: Nur
Merck & Co. Innovates with a Monthly HIV PrEP Pill
In a groundbreaking development, scientists at Merck & Co. have introduced MK-8527, a cutting-edge candidate for HIV pre-exposure prophylaxis (PrEP). Designed to be taken just once a month, this pill aims to significantly reduce the risk of HIV infection and is currently undergoing Phase 3 clinical trials.
Rethinking HIV Prevention: A Look at Current Options
The landscape of HIV prevention has mostly revolved around daily pills like Truvada and Descovy, or bi-monthly injections like Apretude and Yeztugo. However, Merck chemist Izzat T. Raheem emphasizes that flexibility is crucial: 'We need to provide options that help people stick to their PrEP regimens.' MK-8527's convenient monthly dosage could be a game-changer.
The Science Behind MK-8527: A New Class of Antivirals
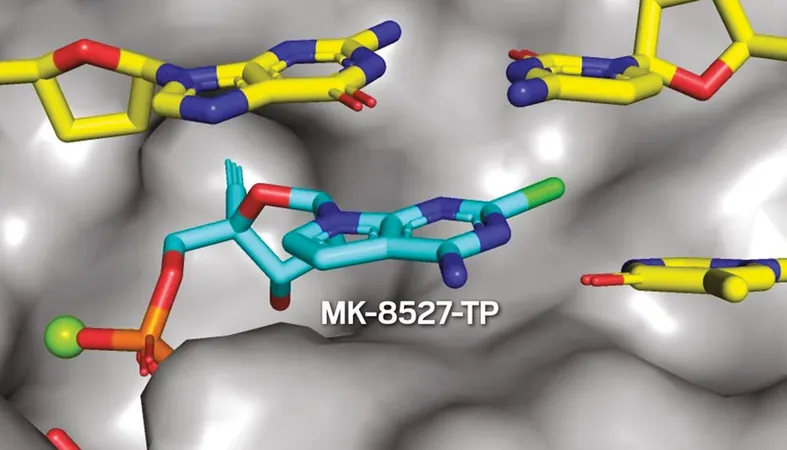
Emerging from Merck’s islatravir program, MK-8527 belongs to a pioneering group of antivirals known as nucleoside reverse transcriptase translocation inhibitors (NRTTIs). This innovative class disrupts HIV's replication processes, providing a potential new line of defense against the virus.
Crafting a New Solution: The Journey to MK-8527
Raheem and his team meticulously explored hundreds of molecular variations to transition from islatravir to MK-8527. They identified two crucial modifications that optimize the compound: substituting nitrogen for carbon in the nucleobase and replacing fluorine with chlorine.
The Alkyne Advantage: A Key Player in HIV Prevention
A standout feature of both molecules is the presence of an alkyne group, essential for thwarting HIV’s replication. As Raheem explains, this alkyne effectively anchors into a specific pocket within the virus's structure, proving instrumental in preventing the translocation process that allows HIV to propagate.
Expert Perspectives: The Future of HIV PrEP
Dennis Liotta, a seasoned expert in HIV therapeutics from Emory University, highlights the promising implications of MK-8527. He notes the pressing need for a more frequent dosing option than existing six-month treatments like lenacapavir, as this could alleviate concerns arising from drug interactions. MK-8527’s monthly regimen strikes a balance between ease of compliance and safety, making it an exciting prospect for HIV prevention.




 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 Česko (CS)
Česko (CS)
 대한민국 (KO)
대한민국 (KO)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)
 الإمارات العربية المتحدة (AR)
الإمارات العربية المتحدة (AR)