Revolutionary Microcellular Drones Target Lung Cancer with Precision Drug Delivery
2024-11-11
Author: Jia
Introduction
In an exciting breakthrough, scientists from the National University of Singapore (NUS) have developed a novel approach to combat lung cancer using microcellular drones—extracellular vesicles (EVs) derived from red blood cells—to deliver powerful anti-cancer drugs directly to tumor sites. This innovative study, led by Assistant Professor Minh Le, represents a significant advancement in the fight against non-small cell lung cancer (NSCLC), particularly for patients who do not smoke.
The Challenge of Lung Cancer
Lung cancer remains the leading cause of cancer-related deaths worldwide, and NSCLC, the most prevalent subtype, poses a particularly challenging problem due to its rapid mutation and development of drug resistance. Traditional treatments, such as tyrosine kinase inhibitors (TKIs), often lose effectiveness as the cancer cells adapt and mutate, leaving patients with limited options.
A Novel Approach with Antisense Oligonucleotides
To address this urgent issue, the research team turned to antisense oligonucleotides (ASOs), which are designed to target specific genetic mutations in cancer cells. This method of precision medicine seeks to tailor treatments to the unique genetic profile of each patient’s cancer, contrasting sharply with older, broad-spectrum therapies that may not effectively combat individual cancers.
The Mechanism of ASOs
ASOs work by binding to specific RNA sequences associated with cancer-related gene mutations, and researchers found that, unlike traditional medications, ASOs could be redesigned to target various genetic anomalies. However, ASOs are prone to degradation in the bloodstream, diminishing their efficacy before reaching the tumor.
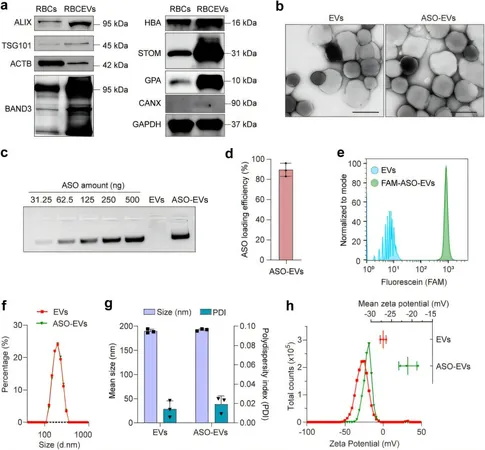
Enhancing Delivery with Extracellular Vesicles
By repurposing extracellular vesicles as delivery vehicles, the scientists were able to enhance the stability and effectiveness of ASOs. These EVs are engineered with particular surface modifications that allow them to hone in on mutant epidermal growth factor receptors (EGFRs), the primary drivers of lung cancer, especially prevalent among Asian populations.
Laboratory Success
In laboratory models of lung cancer, including those derived from patient biopsies, ASO-loaded EVs demonstrated a robust capacity to suppress mutant EGFR activity while leaving healthy cells unharmed. This approach not only provides a smarter method of drug delivery but also offers hope for patients who previously faced limited treatment options due to drug resistance.
Expert Opinions
“Using extracellular vesicles as a natural carrier for delivering anti-cancer ASOs could redefine the landscape of cancer therapies,” expressed Associate Professor Tam Wai Leong from the A*STAR Genome Institute of Singapore. “This method has the potential to be a game-changer in personalized medicine, providing tailored treatment regimens that precisely target cancer cells.”
Conclusion and Future Implications
The research team’s findings, published in *eBioMedicine*, underline a pivotal shift towards more personalized, effective cancer treatment strategies. The ability to algorithmically design ASOs specific to individual patients’ cancer profiles is a monumental step toward overcoming one of the most persistent challenges in oncology.
With the ongoing development of these microcellular drones, the potential implications for other cancers are enormous. As Professor Goh Boon Cher remarked, “This work sets the foundation for similar strategies that could be applied in other forms of cancer treatment, effectively turning the tide against malignancies that have long resisted conventional therapies.”
As the scientific community continues to investigate the full capabilities of these EVs, we may soon witness a transformative era in cancer treatment, where therapies are not only more effective but personalized to the unique genetic makeup of each patient.


 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)