Revolutionary Imaging Technique Unveils Secrets of Bacterial Biofilm Dispersal
2025-05-08
Author: Rajesh
A groundbreaking imaging technique from Carnegie Mellon University is changing the game in microbiology, offering a real-time, high-resolution glimpse of individual bacterial cells as they escape their biofilm communities. This innovative research, published in PLOS Biology, illuminates the dynamics of biofilm formation and dispersal, enhancing our understanding of pathogen spread.
Biofilms are multicellular bacterial communities that provide essential survival advantages, allowing bacteria to share nutrients and fend off threats like antibiotics. Alarmingly, up to 70% of human bacterial infections are attributed to these resilient biofilm-forming bacteria.
Despite being anchored to surfaces, biofilms are not static. Some, like those formed by Vibrio cholerae, undergo cycles of formation and disassembly, enabling free bacteria to disperse into new environments—a pivotal factor in spreading infections.
A Game-Changer in Biofilm Research
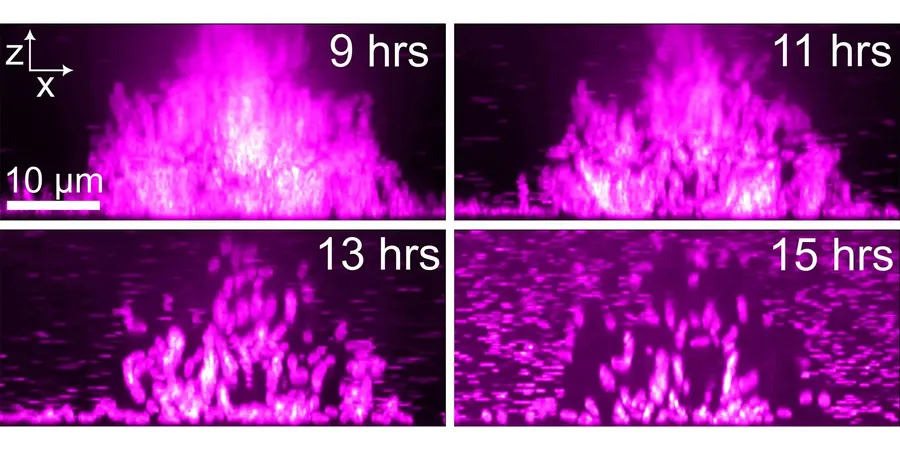
Previous attempts to study biofilm dispersal faced significant challenges due to limitations in imaging technology. Drew Bridges, assistant professor in the Department of Biological Sciences, expressed the frustration of earlier microscopy methods, stating, "No one had been able to image biofilm dispersal with the sort of resolution that we were able to achieve." Enter FAP labeling technology.
Fluorogen-activating proteins (FAPs) are revolutionizing bacterial imaging. They emit fluorescent light only when bound to specific dyes, becoming a powerful tool for scientists. FAPs excel in dense environments like biofilms, where traditional fluorescent proteins falter due to lack of oxygen. Bridges noted, "I learned about FAPs, and they're the perfect alternative because their mechanism is very different from how other fluorescent proteins work."
Illuminating the Unknown
Developed at Carnegie Mellon in 2008, FAP technology has yielded over 150 scientific papers exploring its biological applications. This study marks the first time FAPs have been applied to image biofilms.
Collaborating closely with FAP expert Robert van de Weerd, Bridges' lab integrated FAPs into the Vibrio cholerae genome, utilizing malachite green-derived fluorogens to visualize the bacteria's movement. Using spinning-disk confocal microscopy, the team meticulously tracked how cells within V. cholerae biofilms disassemble and disperse.
New Insights into Bacterial Behavior
The imaging revealed an intriguing pattern: cells primarily dispersed from the biofilm's edges, but approximately 20-25% of cells remained behind. Bridges is delving further to ascertain whether these cells are trapped or engaged in a different role altogether.
Additionally, the research identified dynamic dispersal "hot spots" where bacteria exhibited significant movement. Some peripheral cells compressed towards the biofilm center, suggesting a complex structural interaction. "I hypothesize that cells are a major mechanical component in the biofilm, and their departure leads to structural collapse," Bridges explained.
This research hints at a model where select areas of biofilms behave more fluidly, facilitating the movement of cells even from the interior, while rigid clusters compress to occupy vacant spaces.
What's Next?
The Bridges lab is eager to investigate how these mechanical properties develop throughout biofilm life cycles and intends to apply FAP technology to other notorious biofilm-forming bacteria. This revolutionary imaging technique not only augments our understanding of bacterial dynamics but also holds potential implications for combating biofilm-related infections.



 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 Česko (CS)
Česko (CS)
 대한민국 (KO)
대한민국 (KO)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)
 الإمارات العربية المتحدة (AR)
الإمارات العربية المتحدة (AR)