Revolutionary Hydrogel Technology Opens the Door to Enhanced Drug Delivery with Ultrasound Activation
2024-09-28
Author: Mei
Introduction
In a groundbreaking development, researchers at Michigan Medicine have unveiled a cutting-edge composite hydrogel that employs ultrasound activation to enable sustained and controlled drug release. This innovative approach promises to transform drug delivery methods, offering consistent drug levels that are essential for effective therapeutic outcomes in various medical treatments.
Study Overview
The study, titled "Acoustically responsive scaffolds: Unraveling release kinetics and mechanisms for sustained, steady drug delivery," is featured in the highly regarded October 2024 issue of the Journal of Controlled Release. The researchers have introduced a novel construct known as an acoustically responsive scaffold, which uses a fibrin hydrogel matrix as its foundational element.
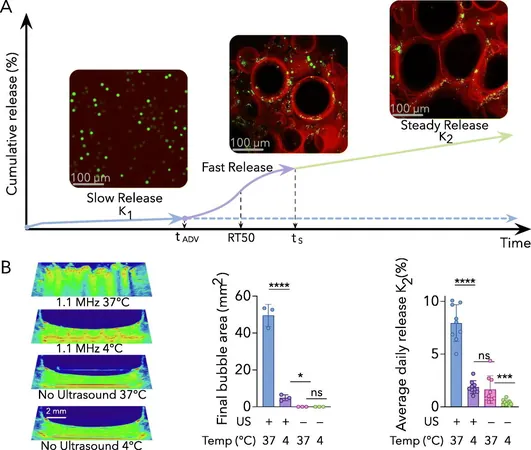
How It Works
But how does it work? When subjected to ultrasound waves, the emulsion embedded within this hydrogel rapidly vaporizes into bubbles, which subsequently release the encapsulated medication. This technique presents a significant advancement over traditional drug delivery systems, such as osmotic pumps that deliver a constant release over time but often come with several limitations.
Benefits of the Hydrogel
Dr. Haijun Xiao, a research fellow at the Michigan Medicine Ultrasound Laboratory and the lead author of the study, highlighted a major benefit of this innovative hydrogel. “We use fibrin, a biocompatible material that naturally degrades within the body. This means that there’s no requirement for surgical removal of the drug delivery device after treatment, contrary to what is needed with many existing implantable systems.”
Controlled Release Mechanism
The ability to finely tune ultrasound exposure allows for a steady, zero-order release of medication, which translates to a uniform drug level over prolonged periods. Dr. Xiao emphasized the significance of this aspect, stating, “This controlled release not only enhances treatment effectiveness but also reduces the side effects that can result from fluctuating drug concentrations.”
Mathematical Model
To further illustrate the release dynamics, Dr. Xiao developed complex equations that define the multi-phasic release behavior of the scaffolds. The process initiates with a prompt release triggered by ultrasound, followed by a sustained release phase that maintains steady drug levels.
Implications and Future Directions
The implications of this technology are vast. The Michigan Medicine Ultrasound Laboratory has previously utilized these scaffolds for stimulating angiogenesis, or blood vessel growth. By applying this advanced technology to drug delivery systems, the researchers are paving the way for tailored treatment plans that allow for on-demand release and non-invasive adjustments to dosages.
"As we move forward, having a mathematical model that accurately represents the release process from these scaffolds will be vital for personalizing treatments,” said Dr. Mario L. Fabiilli, principal investigator at the Ultrasound Laboratory and senior author of the study. “These equations will enable us to precisely optimize drug dosages in a non-invasive manner, catering to the unique needs of each patient.”
Conclusion
This innovative hydrogel technology could soon redefine how medications are delivered, making treatments not only more effective but also more convenient for patients. Keep an eye on Michigan Medicine as they continue to break new ground in medical research and drug delivery technologies!



 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)