Revolutionary High-Entropy Nanoribbons: The Game-Changer for Extreme Conditions
2025-05-29
Author: Wei Ling
In a groundbreaking development, a research team from Southern Methodist University (SMU) has unveiled a cutting-edge material known as high-entropy oxide (HEO) nanoribbons. These innovative nanoribbons are set to transform industries by offering unparalleled resistance to heat, corrosion, and other harsh environmental conditions, outperforming traditional materials.
Featured in the esteemed journal Science, these remarkable HEO nanoribbons present exciting possibilities in fields such as aerospace, energy, and electronics. They excel in extreme conditions where conventional materials often falter.
What sets these nanoribbons apart is their manufacturing process that allows for 3D-printing or spray-coating at room temperature, marking a significant advancement in energy-efficient and cost-effective production methods. Unlike prior high-entropy materials that necessitated high-temperature casting and existed primarily as bulk forms, the ability to craft these nanoribbons at room temperature heralds a new era in material science.
Understanding High-Entropy Oxide (HEO) Nanoribbons
So, what exactly are high-entropy oxide nanoribbons? Imagine them as ultra-thin stripes of material, often just a few nanometers thick, with widths ranging from tens to hundreds of nanometers. These nanoribbons fall under the category of high-entropy materials, which are characterized by a high degree of disorder within their atomic structure.
A fitting analogy is a fruit salad made not just with grapes but with a rich variety of fruits—all equally represented. High-entropy materials function on the same principle, blending five or more elements in nearly equal proportions. This diversity results in enhanced strength, heat resistance, and superior durability against stress or corrosion.
The Future of Material Science
Under the guidance of lead researcher Amin Salehi-Khojin and collaborators from the University of Illinois Chicago, Stockholm University, and the University of Washington, a novel approach has been developed to produce these low-dimensional high-entropy materials economically and sustainably.
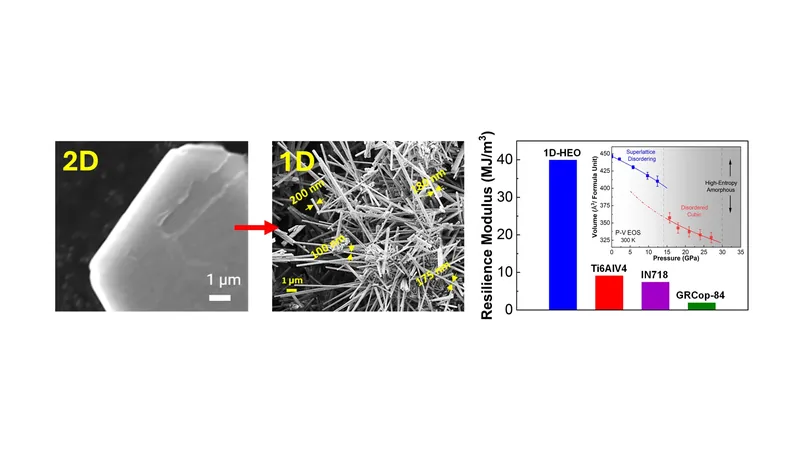
Co-author Ilias Papailias, a Research Assistant Professor at SMU's Mechanical Engineering Department, elaborated on the innovative synthesis method. By utilizing sulfur to etch two-dimensional structures from the high-entropy materials, followed by an oxidation process, they were able to convert these into one-dimensional nanoribbons. This new technique offers exceptional control over the size and width of the resulting nanoribbons, allowing for precise manufacturing.
The research demonstrates that these newly crafted nanoribbons, branded as 1D-HEO, can withstand extreme temperatures reaching up to 1,000 °C, high pressures of up to 12 gigapascals, and remain resilient in harsh chemical environments (pH levels ranging from 2.3 to 13 for seven days).
Though further testing is necessary before widespread application, the properties of 1D-HEO point to its suitability for applications requiring exceptional heat resistance, pressure tolerance, and durability under intense mechanical stress. Salehi-Khojin asserts that this pioneering method could revolutionize materials science by introducing unprecedented entropy structures.



 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 Česko (CS)
Česko (CS)
 대한민국 (KO)
대한민국 (KO)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)
 الإمارات العربية المتحدة (AR)
الإمارات العربية المتحدة (AR)