Revolutionary Glow Assay Transforms Detection of Disease-Causing Mutations
2025-09-02
Author: Mei
Unlocking the Secrets of Protein Mutations
In the world of genetic research, not every mutation spells disaster. Just as not every clue at a crime scene leads to a culprit, some mutations in proteins can be harmless. However, differentiating between these benign changes and those that trigger diseases is a daunting task. This is where a new glow-based assay comes into play, promising to illuminate the mysteries surrounding protein mutations.
A Game-Changer in Protein Analysis
While current methods like mass spectrometry, nuclear magnetic resonance (NMR), and protein gels have their uses, they often come with high costs or limited output. Researchers at the University of Michigan, led by Michael Wang, have unveiled a groundbreaking assay published in the Journal of Biological Chemistry that simplifies the process dramatically. This innovative tool is quick, easy to execute, and delivers quantifiable results, making it ideal for high-throughput screening.
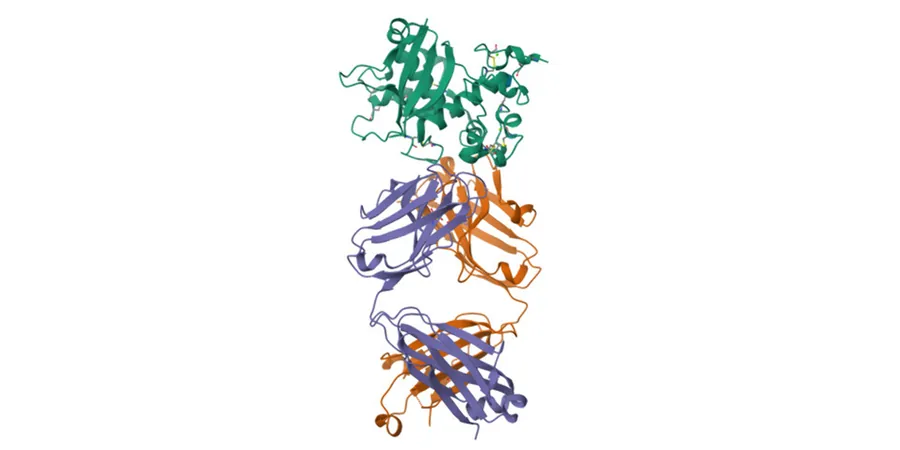
Spotlighting NOTCH3 and CADASIL
The study focused on the NOTCH3 protein, a vital membrane-bound receptor crucial for vascular health. Over 200 mutations in NOTCH3 are linked to CADASIL, a rare genetic disorder that leads to debilitating strokes and dementia. Yet, not every mutation is a direct contributor to the disease. Understanding which alterations are truly pathogenic is essential for effective diagnosis and treatment.
How the Glow Assay Works
Wang and his research specialist, Naw May Pearl Cartee, ingeniously redesigned a split-luciferase assay. They split a glowing luciferase molecule into two parts, strategically placing these halves on opposite sides of the NOTCH3 protein. The proximity of these halves, determined by the protein's structure, dictates whether they glow. By quantifying this glow, they can assess changes in the protein structure.
More Than Just Glow – Understanding Protein Behavior
This assay isn't just a flashy trick; it can also measure how mutations influence protein behavior, particularly in their secretion and trafficking. By evaluating numerous NOTCH3 mutations, the duo found that changes in cysteine count significantly disrupt the protein’s structure. Interestingly, restoring an even number of cysteines mitigates these negative effects, hinting at pathways for improved diagnostic strategies.
Ease of Use – A Boon for Researchers
One of the standout features of this assay is its simplicity. "It’s a straightforward process," Pearl noted. Researchers can quickly transfect the cells and in just a day, start observing results. This speed makes it an efficient tool for scouring through vast numbers of mutations.
Rank, Don’t Just Identify
Unlike conventional binary assays that merely indicate whether a mutation is harmful or not, this glow assay allows researchers to rank the severity of mutations. Additionally, it detects whether NOTCH3 is correctly folded, offering potential insights into therapeutic developments. Identifying compounds that can restore proper protein structure could lead to revolutionary treatments for cysteine-related diseases.
A Bright Future Ahead
Wang expressed hopes that researchers tackling cysteine-associated conditions will adopt this assay widely. With its impressive capabilities, this new glow-based tool is set to revolutionize the way scientists detect and understand disease-causing mutations, shining a light on previously hidden paths in genetic research.


 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 Česko (CS)
Česko (CS)
 대한민국 (KO)
대한민국 (KO)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)
 الإمارات العربية المتحدة (AR)
الإمارات العربية المتحدة (AR)