Revolutionary EIT System Now Approved for Premature Infants in the U.S.
2025-09-04
Author: Daniel
Breakthrough in Neonatal Care: The LuMon™ EIT System
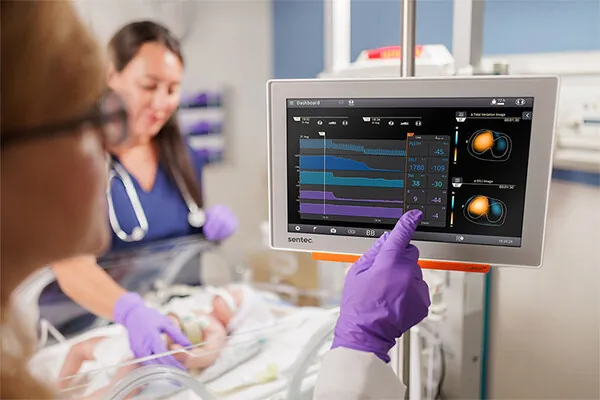
In a groundbreaking development for neonatal health, Sentec has announced that the FDA has granted 510(k) clearance for its LuMon™ Electrical Impedance Tomography (EIT) system. This innovative technology is now the first of its kind in the United States, specifically approved for use with premature infants and spontaneously breathing patients.
Transforming Bedside Monitoring
The LuMon™ EIT system provides real-time lung imaging right at the bedside without any radiation exposure. This capability allows healthcare providers to tailor treatment more effectively to the unique needs of each patient, particularly in critical care settings. Neonatologists are especially excited by its potential.
Personalized Care for Fragile Lives
Dr. David Tingay, a Clinical Neonatologist at the Royal Children’s Hospital in Melbourne, emphasizes the complexity involved in ventilating neonatal patients. He states, "In our smallest patients, there is little room for error, making precise monitoring crucial to their long-term health." With the LuMon™ system, clinicians can continuously visualize lung function, gaining insights that were previously unattainable.
Why This Technology Matters
The LuMon™ system is not only significant because it offers the first EIT technology approved for premature infants; it includes soft, fabric belts that are small enough for very low birthweight babies. This advancement means that neonatal intensive care units (NICUs) can provide more gentle and effective respiratory care.
Understanding the Science Behind EIT
Electrical Impedance Tomography works by sending gentle, alternating currents through a lightweight, skin-friendly belt placed around the thorax. It measures the resulting voltages to create dynamic images that reflect lung function in real-time, helping clinicians adjust treatment strategies based on immediate feedback.
A Commitment to Innovative Care
Founded in 1999, Sentec has been at the forefront of respiratory care technology, combining cutting-edge science with compassionate healthcare solutions. Their commitment to non-invasive and continuous monitoring positions the LuMon™ system as a game-changer in the pursuit of better patient outcomes.
Look Out for This Life-Changing Innovation
With the introduction of the LuMon™ EIT System, clinicians now have a potent tool at their disposal. This FDA-cleared device is set to revolutionize how healthcare providers manage neonatal respiratory care, offering new hope for the tiniest patients.

 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 Česko (CS)
Česko (CS)
 대한민국 (KO)
대한민국 (KO)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)
 الإمارات العربية المتحدة (AR)
الإمارات العربية المتحدة (AR)