Revolutionary Discovery: Scientists Unearth ‘Lipocartilage’ with Game-Changing Potential for Regenerative Medicine!
2025-01-14
Author: Siti
Introduction
In a groundbreaking study that could reshape the future of regenerative medicine and tissue engineering, a team of international researchers, spearheaded by experts from the University of California (UC) Irvine, has identified a newly discovered skeletal tissue dubbed “lipocartilage.” This remarkable tissue, found in the ears, noses, and throats of mammals, boasts properties that offer extraordinary resilience and flexibility, opening the door to innovative treatments for various medical conditions.
Key Attributes of Lipocartilage
Senior author Maksim Plikus, PhD, a noted professor of developmental and cell biology at UC Irvine, praised the exceptional qualities of lipocartilage, stating, “Its resilience and stability provide compliant, elastic capabilities ideal for flexible body parts such as earlobes or the tip of the nose. This discovery paves the way for exciting advancements in regenerative medicine, especially for reconstructing facial defects and injuries.”
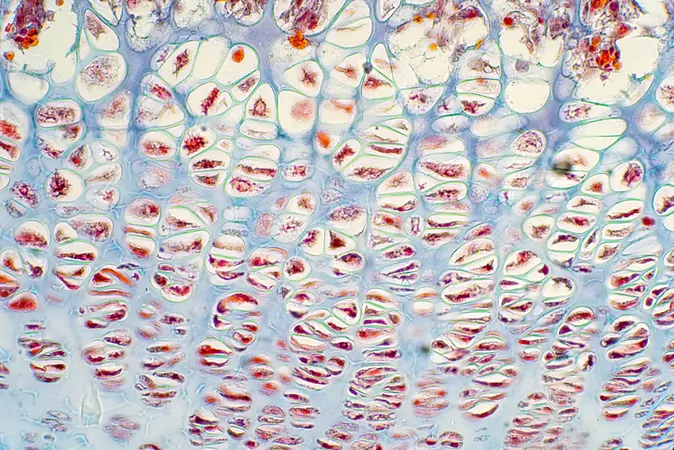
Composition and Structure
Published in the prestigious journal *Science*, the research reveals that lipocartilage consists of specialized fat-filled cells known as “lipochondrocytes.” These unique cells serve as a stable internal support system, allowing the tissue to remain soft and pliable—akin to the cushioning bubbles found in packing materials. Unlike traditional fat cells, lipochondrocytes maintain fixed lipid reservoirs, preserving their size even when food sources fluctuate. This characteristic is vital for maintaining tissue strength and flexibility.
Distinguishing Features
What sets lipocartilage apart from standard cartilage is its innovative framework. Traditional cartilage relies heavily on an external extracellular matrix for structural support, whereas lipocartilage boasts a remarkable internal stability brought about by its lipochondrocytes. These cells produce lipid-filled vacuoles that are "locked" in place through a special genetic process, effectively halting the breakdown of fats. This novel understanding of lipids in skeletal tissues is poised to make significant impacts on the field of tissue engineering.
Gene Expression and Metabolic Processes
Furthermore, the researchers discovered that precursor cells of lipocartilage express gene combinations typically associated with both cartilage and fat cells. Through the process known as de novo lipogenesis, these cells generate lipid vacuoles from glucose, distinguishing them from adipocytes, which derive fat from the bloodstream. Even under varying diets or caloric intake, lipocartilage remains constant in size—a stark contrast to traditional fat networks that expand and contract according to metabolic shifts.
Future Directions and Implications
Raul Ramos, the lead author of the study and a postdoctoral researcher in the Plikus lab, noted the promising future directions of this research, emphasizing the importance of understanding how lipochondrocytes maintain their stability over time, and exploring the molecular pathways involved in their function and development. Moreover, insights gained from this research could yield valuable knowledge about cellular aging.
Observations in Mammals
Intriguingly, the research team also observed that certain mammals, such as bats, exhibit intricately arranged lipocartilage in their ears, structured in parallel ridges that enhance their auditory capabilities by modulating sound waves. In a significant leap towards human applications, lipocartilage was identified in human cartilage cells cultivated from embryonic stem cells, igniting hopes for its potential usability in medical therapies.
Potential for Regenerative Medicine
As researchers dive deeper into the molecular mechanics that sustain lipocartilage's stability, they are eyeing its applications in regenerative medicine. The advancement of 3D printing technologies could soon allow for the creation of personalized, living cartilage tissues tailored to meet individual medical needs.
Conclusion
With these promising developments, the study holds the potential to revolutionize how we approach reconstructive surgeries. Current practices often necessitate the harvesting of cartilage from a patient's rib, a method that can be invasive and painful. However, the possibility of generating customized lipocartilage tissue from stem cells may lead to less invasive and more personalized treatments for cartilage defects, trauma, and degenerative diseases.
Final Remark
Stay tuned as the world watches these developments unfold—this discovery could very well be the key to unlocking new therapeutic avenues in medicine!


 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 Česko (CS)
Česko (CS)
 대한민국 (KO)
대한민국 (KO)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)
 الإمارات العربية المتحدة (AR)
الإمارات العربية المتحدة (AR)