Revolutionary Discovery: New Insights into Mitochondrial Protein Import
2025-08-29
Author: Rajesh
Unlocking the Secrets of Mitochondria
Mitochondria, often called the powerhouses of the cell, generate ATP (adenosine triphosphate), the energy currency that fuels cellular functions. Originally, these organelles emerged over a billion years ago through a remarkable symbiotic relationship between primitive archaeal cells and ancestral bacteria. Over millennia, they evolved to rely heavily on their host cells for protein supplies and lost most of their DNA.
Caltech's Groundbreaking Research
Caltech scientists have recently shed light on the intricate process of how mitochondrial proteins are imported from ribosomes in the cytosol—where protein synthesis occurs—to the mitochondria themselves. In a fascinating turn of events, this process is significantly influenced by the complexities of protein folding.
Shu-ou Shan, the Altair Professor of Chemistry at Caltech, states, "Localizing proteins to mitochondria involves a multilayered, complex pathway intricately linked to the biophysical principles of protein folding." This new discovery challenges the long-held view that mitochondrial proteins are solely imported after their synthesis is complete.
A Shocking Revelation: Cotranslational Import
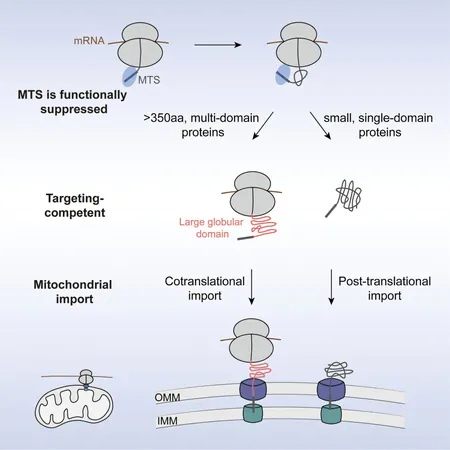
In an enlightening paper published in the journal Cell, Shan and her team reveal that up to 20% of mitochondrial proteins can be imported cotranslationally—meaning that they enter the mitochondria while still being formed by ribosomes.
Lead author Zikun Zhu (Ph.D. '24) highlights the uniqueness of these proteins shortly after identifying them. He explains, "What is special about this subset of proteins?" The answer lies in their complexity. These proteins are significantly larger and require intricate folding processes, involving interactions that extend beyond immediate neighboring amino acids.
The Challenge of Folding
The complexity of these topologically rich proteins creates significant challenges during import. If large proteins complete their synthesis in the cytosol, they risk forming irreversible structures that could obstruct mitochondrial channels. Shan suggests, "Large, complex proteins could get stuck, blocking the channels needed for import."
How Does The Cell Decide?
But what guides the cell in determining which proteins need to be imported during translation? The research team discovered that nearly all these proteins possess a mitochondrial targeting sequence, a signal for directing them to mitochondria. Surprisingly, this sequence alone isn't sufficient to initiate cotranslational import.
Zhu’s experiments unveiled a second crucial molecular signal: the emergence of the first large protein domain. He metaphorically describes this process, stating, "It's like having your boarding pass locked in a suitcase. The targeting sequence is your boarding pass, but you need the code to open the suitcase, which is the large domain."
Implications for the Future
The team was able to transplant these large domains into other mitochondrial proteins that typically undergo post-translational import, demonstrating that this structural feature serves as a transferable signal for early import. Zhu remarks, "Cotranslational targeting to mitochondria is completely different from targeting to other organelles." The implications of this discovery are immense: as researchers further uncover the mechanisms behind mitochondrial protein import, it could pave the way for groundbreaking therapeutic applications, shedding light on why cells developed such a sophisticated system and how it can be harnessed for medical advancements.




 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 Česko (CS)
Česko (CS)
 대한민국 (KO)
대한민국 (KO)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)
 الإمارات العربية المتحدة (AR)
الإمارات العربية المتحدة (AR)