Revolutionary Discovery: How Superoxide Powers Enzyme-Driven Drug Synthesis!
2025-03-25
Author: Sarah
Introduction
Enzymes, often called the catalysts of life, are vital for numerous biological activities including metabolic regulation and energy transduction. These remarkable molecular machines, honed by billions of years of evolution, not only play essential roles in biological systems but also present invaluable tools in the realm of synthetic biology. They can transcend the constraints of conventional chemical synthesis, acting as micro-factories that dramatically enhance the production of antibiotics, biofuels, and other valuable compounds.
Study Overview
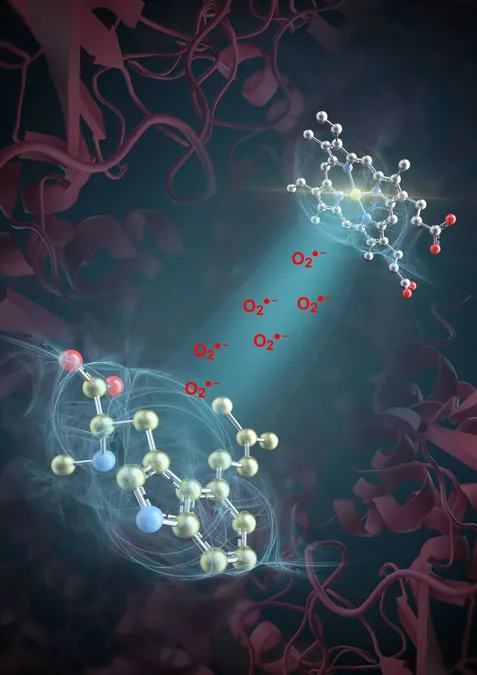
A groundbreaking study conducted by scientists at the Tianjin Institute of Industrial Biotechnology, part of the Chinese Academy of Sciences, alongside their colleagues from Hangzhou Normal University, has revealed a new facet of enzymatic mechanisms. Published in the esteemed journal *Nature*, this research uncovers the catalytic role of superoxide (O2•-), a reactive oxygen species (ROS), specifically within the heme-catalase-mediated synthesis of ergot alkaloids (EAs)—medicinal compounds known for their effectiveness against a variety of diseases.
Dual-Catalytic Mechanism
In this innovative study, the researchers focused on the enzyme EasC, which is integral to the biosynthesis of ergot alkaloids. They discovered a fascinating dual catalytic mechanism inside EasC composed of two specialized "workshops.” One workshop is nestled within the enzyme's heme pocket, responsible for generating superoxide, while the second workshop resides on the surface pocket, linked by a narrow tunnel facilitating efficient transit of the superoxide. This unique setup allows EasC to utilize the activity of superoxide to catalyze reactions that produce ergot alkaloids.
Nature's Ingenious Design
This ingenious dual-workshop design, coupled with a dedicated transport pathway, illustrates nature's ability to harness the reactivity of ROS without succumbing to its damaging effects. Unlike previous beliefs that producing superoxide required external electron donors, this study highlights a novel mechanism where the necessary reduction of oxygen is performed by the enzyme’s substrate itself.
Superoxide: A Double-Edged Sword
Interestingly, while superoxide is often vilified for its potential to harm DNA, proteins, and other cellular components, this research shines a light on its constructive role in biosynthesis. The revelation proves that even molecules typically seen as detrimental can be cleverly repurposed in the intricate dance of biochemical pathways.
Pharmaceutical Implications
Beyond its scientific significance, the implications of this discovery are poised to resonate throughout the pharmaceutical industry. With clinical advancements like lysergic acid diethylamide (LSD) receiving breakthrough therapy designation from the FDA in 2024 for generalized anxiety disorder, the relevance of ergot alkaloids is clearer than ever.
Future Directions
As researchers continue to unravel the intricacies of enzyme-driven drug synthesis, the findings from this study could revolutionize the production of ergot alkaloids. They may pave the way for developing engineered cell factories that enable sustainable and eco-friendly pharmaceutical manufacturing, positioning the industry towards greener practices and reduced carbon footprints.
Conclusion
Will this discovery redefine how we approach drug synthesis? Stay tuned as the world watches how these revelations could turn the tide in pharmaceutical production!


 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 Česko (CS)
Česko (CS)
 대한민국 (KO)
대한민국 (KO)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)
 الإمارات العربية المتحدة (AR)
الإمارات العربية المتحدة (AR)