Revolutionary Discovery: How Scientists Are Cracking the Code of Skin Cancer Treatment!
2024-09-23
Groundbreaking Discovery at UCLA
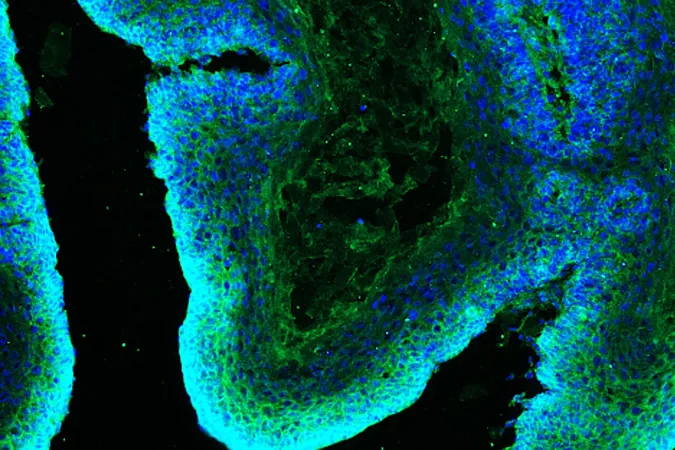
Scientists at the renowned Eli and Edythe Broad Center of Regenerative Medicine and Stem Cell Research at UCLA have made a groundbreaking discovery regarding the metabolic strategies employed by squamous cell skin cancers to evade treatment. This pivotal research, published in Science Advances, unveils new avenues to potentially halt cancer progression and reshape therapeutic approaches.
Challenging the Warburg Effect
The lead researcher, Professor William Lowry, has previously challenged the long-standing Warburg effect theory, which posited that cancer cells primarily utilize glucose for their energy needs. Contrary to this notion, Lowry's team revealed that squamous cell skin cancer cells exhibit remarkable metabolic flexibility—they can switch their energy source from glucose to glutamine when resources are scarce.
"Previous clinical approaches targeting cancer metabolism failed largely because they addressed one pathway at a time," explained Lowry. "In reality, tumors have access to multiple nutrients, enabling them to adapt and survive."
Investigating Metabolic Flexibility
Digging deeper into this metabolic flexibility, graduate student Carlos Galván spearheaded further investigations using mouse models. Attempting to cut off the supply of glutamine—an integral nutrient for these cancer cells—Galván discovered that the tumors merely adapted by shifting to alternative energy sources. "It's just like a game of whack-a-mole!" he noted. "When you block one pathway, the cancer cells discover a new nutrient to exploit."
Dual-Targeting Strategy
To combat this resilient trait, the team employed a dual-targeting strategy, blocking both glucose and glutamine metabolism. Remarkably, this intensive approach was sufficient to halt tumor growth in their models.
Mechanisms of Adaptation
Galván's findings also revealed that this metabolic adaptability is not primarily orchestrated by changes in gene expression but instead involves a swift reorganization of transporter proteins at the cell membrane, facilitating rapid nutrient uptake.
Towards Clinical Applications
As the researchers gear up for clinical applications, they are currently working on developing pharmacological inhibitors aimed at these critical enzymes involved in the cancer's metabolic mechanisms. An exciting prospect is a topical therapeutic approach—which would allow direct application to skin tumors, potentially enhancing treatment efficacy while minimizing systemic side effects.
"The beauty of targeting skin cancer lies in its accessibility," Galván emphasized. "Topical treatments could prove more effective and safer, yet challenges remain, including ensuring that the drug penetrates the skin and retains its effectiveness over time."
Implications Beyond Skin Cancer
While focusing on squamous cell skin cancer, the implications of this research extend far beyond, as many other cancers exhibit similar metabolic behaviors. The team is already exploring how these strategies could be applied to diseases like melanoma.
Continued Research Efforts
Additionally, their research continues to delve into the intricacies of protein regulation and metabolic flexibility in cancer cells. "If we can understand how these cells sense and adapt to metabolic stress, we might identify ways to disrupt their adaptability—an exciting potential alternative to merely blocking transporters," Lowry noted.
Conclusion: Hope for Cancer Treatment
This promising research not only signals hope for enhanced treatment options for skin cancer patients but also opens doors to innovative strategies that could disrupt cancer metabolism across a wide spectrum of malignancies. Stay tuned as scientists strive to unlock the mysteries of cancer and develop life-saving therapies!

 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)