Revolutionary Discovery: How Phosphorylation of Tau Protein Could Unlock Secrets of Early Alzheimer's
2025-04-17
Author: Li
A Breakthrough Study on Tau Protein and Alzheimer's Disease
In a groundbreaking study published in the Journal of the American Chemical Society, scientists have unveiled how a critical brain protein, tau, morphs into a clumping agent when a phosphate group attaches to it—a process known as phosphorylation. This alteration is pivotal as tau normally plays a role in maintaining healthy brain cells, but its abnormal aggregation is implicated in neurodegenerative conditions like Alzheimer's.
Phosphorylated Tau vs. Regular Tau: What’s the Difference?
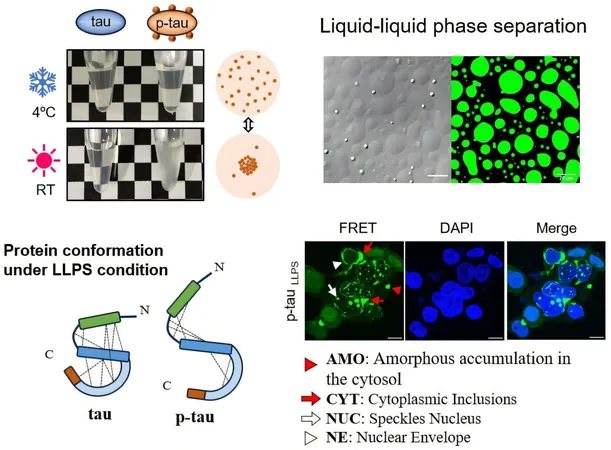
The research team conducted a fascinating comparison between two distinct forms of tau: the phosphorylated version (p-tau) and its non-phosphorylated counterpart. To study p-tau, they ingeniously engineered bacteria to produce this variant using a sophisticated design called PIMAX.
The Mystery of Droplet Formation: How Temperature Changes Everything
Remarkably, they discovered that it’s only p-tau that can spontaneously form tiny droplets—measuring in micrometers—via a process reminiscent of oil separating from water, known as liquid-liquid phase separation (LLPS). This droplet formation occurs naturally when the temperature shifts from cold to room temperature, driven by intricate electrostatic and hydrophobic interactions. Even more astonishing is that these droplets vanish upon cooling—hinting at a reversible process.
A Few Phosphates Go a Long Way!
Further investigations indicated that the addition of just a handful of phosphate groups—from two to nine—was sufficient to enhance tau's flexibility, enabling it to form these droplets.
RNA's Role: A Double-Edged Sword?
In an intriguing twist, researchers found that RNA can also prompt droplet formation for both tau and p-tau. However, unlike the reversible nature of p-tau’s self-coacervation, the droplet formation caused by RNA is irreversible and unfolds on a completely different timeline.
Distinct Aggregation Patterns: A Navigational Clue?
Surprisingly, neither tau nor p-tau on their own led to the formation of amyloid fibrils. Instead, external triggers such as dextran sulfate or RNA were necessary. The study revealed that while various conditions—such as dextran sulfate-induced tau/p-tau fibrils and RNA-induced fibrils—resulted in tau fragment aggregation, only the special conditions surrounding p-tau directly caused aggregation near the nucleus of biosensor cells. This cellular activity bears a striking resemblance to patterns observed in the brains of Alzheimer's patients, providing valuable insights into the disease's origins.
A View Toward Future Research
"This is a significant breakthrough," emphasized Prof. Rita P.–Y. Chen. "Our findings reveal how a simple chemical modification—phosphorylation—can drastically change tau's cellular behavior, opening new avenues for understanding the early mechanisms of Alzheimer's disease." She highlighted the current challenge in neuroscience: the lack of a reliable mouse model that accurately reflects the human condition and announced efforts to develop a new model that encapsulates the pathological features typically seen in Alzheimer's patients.



 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 Česko (CS)
Česko (CS)
 대한민국 (KO)
대한민국 (KO)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)
 الإمارات العربية المتحدة (AR)
الإمارات العربية المتحدة (AR)