Revolutionary Discovery: How Our Cells Repair Mitochondria and Combat Aging!
2025-04-07
Author: Yu
Introduction
Research has unveiled a groundbreaking mechanism that cells use to repair their mitochondria—the powerhouse of the cell—critical for maintaining health and preventing serious diseases like Parkinson's, Alzheimer's, amyotrophic lateral sclerosis (ALS), cardiovascular diseases, and type 2 diabetes. Damage to mitochondrial DNA (mtDNA) not only contributes to these illnesses but also accelerates the aging process.
The Discovery
A dedicated team of scientists from University Hospital Düsseldorf, Heinrich Heine University (HHU), the University of Cologne, and the Center for Molecular Medicine Cologne (CMMC) recently published their findings in the journal *Science Advances*. Led by Professor Pla-Martín from HHU's Institute of Biochemistry and Molecular Biology, the researchers discovered a specialized recycling system that cells engage when they detect mtDNA damage.
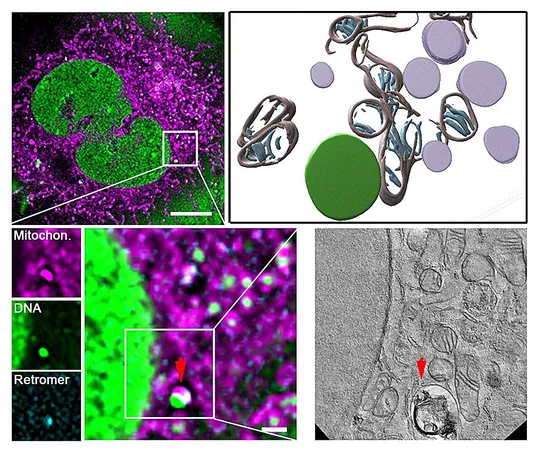
The Mechanism
Their studies reveal that this repair mechanism hinges on a crucial protein complex known as retromer, alongside lysosomes—organelles packed with digestive enzymes that function as the cell's recycling centers. These elements work in concert to remove damaged genetic material, thus preventing the harmful accumulation of faulty mtDNA.
Importance of the Research
"This previously unidentified cellular pathway is vital for maintaining mitochondrial health and enhancing our cells' natural defenses," states Professor Pla-Martín. Understanding this newly discovered mechanism provides insight into how mitochondrial damage can lead to debilitating diseases, opening avenues for developing preventive therapies aimed at tackling such conditions.
Further Research
In a step further, Professor Pla-Martín collaborated with Dr. Parisa Kakanj, a cell biologist at the University of Cologne and a member of the CEPLAS Cluster of Excellence, to expand upon their initial findings using fruit flies (Drosophila) as a model organism. Their research demonstrated that boosting the activity of the retromer complex—specifically the protein VPS35—significantly accelerates the elimination of damaged mitochondrial DNA and enhances overall mitochondrial function.
Implications for the Future
Dr. Kakanj emphasized the importance of these findings, stating, "Using Drosophila allowed us to validate our results in human cells and witness remarkable improvements in mitochondrial health. This discovery opens up thrilling possibilities for therapeutic strategies targeting mitochondrial diseases and age-related conditions."
Conclusion
This research not only sheds light on how cells maintain their energy-producing organelles but hints at a future where targeting mitochondrial repair processes could become a cornerstone of therapies that mitigate the effects of aging and related diseases. Stay tuned, as this breakthrough could revolutionize how we approach mitochondrial health and longevity!

 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 Česko (CS)
Česko (CS)
 대한민국 (KO)
대한민국 (KO)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)
 الإمارات العربية المتحدة (AR)
الإمارات العربية المتحدة (AR)