Revolutionary Discovery: Genetic Fingerprint in Bacteria Could Transform the Fight Against Drug Resistance!
2025-01-28
Author: Mei
Antibiotic resistance is not just a medical nuisance; it's a looming global crisis that each year claims the lives of over a million individuals. Alarmingly, projections by the World Health Organization suggest this issue could eclipse cancer and heart disease, becoming the leading cause of death by 2050 as bacteria evolve and develop resistance to the very drugs designed to eliminate them.
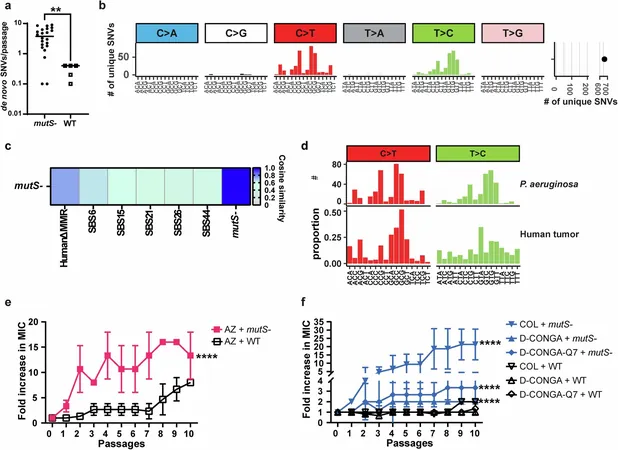
In a groundbreaking study published in *Nature Communications*, researchers from Tulane University have discovered a unique genetic signature within bacteria that can predict their likelihood of becoming resistant to antibiotics. This pivotal finding holds the potential to aid medical professionals in quickly identifying targeted, effective treatments against stubborn and deadly pathogens that boast a track record of drug resistance.
"If we recognize this mutational pattern during genome sequencing, it serves as a warning sign that the bacteria is likely to develop resistance to antibiotic treatment," stated Kalen Hall, Ph.D., the lead author of the study who conducted this pioneering research prior to graduating from Tulane University School of Medicine in 2024.
Central to this study is the notorious *Pseudomonas aeruginosa*, a strain of bacteria notorious for its multidrug resistance that critically endangers patients in hospital settings. Researchers revealed that these bacteria often exhibit deficiencies in a specific DNA repair pathway, a flaw that accelerates mutations—ultimately increasing their chances of developing resistance.
Utilizing a strategy typically reserved for oncology, the research team analyzed bacterial genomes for mutational signatures, leading to the identification of a unique pattern linked to the aforementioned DNA repair deficiencies. This pattern is capable of predicting which bacterial strains may become resistant to antibiotics.
"This fingerprint is essentially a forecast of potential multidrug-resistant bacteria," explained Zac Pursell, Ph.D., an associate professor of biochemistry and molecular biology at Tulane.
Compounding the challenge, the research found that bacteria can acquire resistance not only to the antibiotics initially used but also to other drugs administered afterward. "Over 50% of prescribed antibiotics are either unnecessary or incorrect treatments," Hall stressed. "When the wrong antibiotic is given, it only exacerbates the growing resistance problem."
What’s even more encouraging is that the cutting-edge DNA sequencing technology employed in this study has the dual capability to not only predict bacterial resistance but also pinpoint specific targets for intervention. By identifying different resistance pathways, clinicians can administer tailored antibiotic combinations that thwart resistance development.
While these findings are still in their nascent stages, the development of diagnostic tools based on this research could dramatically reduce the overprescription of antibiotics, paving the way for more accurate treatment protocols against invasive bacterial infections. Hall, who has transitioned into the role of CEO and cofounder of Informuta Inc., a San Diego-based startup, is focused on advancing a machine learning model designed to analyze bacterial samples and forecast antibiotic resistance.
"This innovation represents a paradigm shift in treatment strategies, and it could revolutionize care for countless patients. As antibiotic resistance escalates year after year, adopting precise diagnostics and responsible antibiotic practices has never been more crucial," Hall declared.
Stay informed as this research evolves; the future of effective antibiotic treatment could hinge on these exciting developments in bacterial genetics!

 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 Česko (CS)
Česko (CS)
 대한민국 (KO)
대한민국 (KO)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)