Revolutionary Discovery Could Transform ALS Treatment: The Secret Lies in Tiny Cellular Antennas
2024-12-20
Author: Sarah
Introduction
Amyotrophic lateral sclerosis (ALS), often known as Lou Gehrig's disease, is a crippling neurodegenerative condition that primarily targets motor neurons. This disease can drastically reduce life expectancy to just two to five years post-diagnosis, and there remains a stark lack of effective treatments or a cure. However, an exciting breakthrough from researchers at KU Leuven and the VIB Center for Brain Research may shine a new light on potential therapeutic pathways. Their study, published in the prestigious journal *Brain*, identifies tiny, antenna-like structures on cells called cilia as critical players in the onset and progression of ALS.
Understanding ALS
ALS is recognized as the most prevalent motor neuron disease among adults. It primarily endangers motor nerve cells—those vital cells tasked with transmitting messages from the brain to the muscles for movement. As ALS progresses, these cells deteriorate, leading to debilitating symptoms including paralysis, speaking difficulties, and complications in swallowing and breathing. Tragically, most patients succumb to this relentless disease within a few years of their first symptoms.
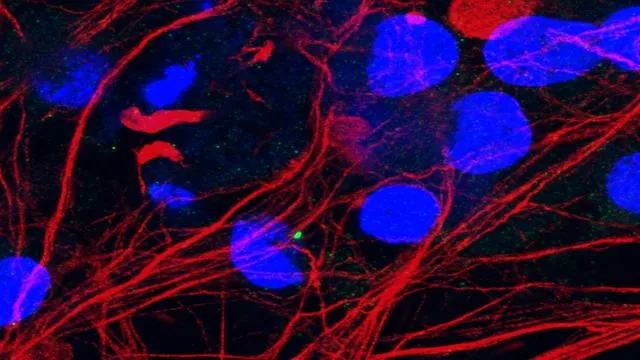
Research Insights
Despite extensive research endeavors over the years, scientists have struggled to pinpoint the definitive cause behind the death of motor neurons. However, recent research suggests a promising correlation with cilia—microscopic structures that function as antennas, crucially responsible for receiving and processing numerous cellular signals.
C21orf2 Gene Discovery
In a pivotal study dating back to 2016, an international consortium led by Prof. Philip Van Damme, a neurologist at UZ Leuven, uncovered the C21orf2 gene as a newly identified ALS-associated gene. Prior investigations had already revealed that mutations in C21orf2 could disrupt cilia function in various diseases. This sparked the researchers' interest in examining whether this gene's influence extended to ALS as well.
Ciliary Dysfunction in ALS
Collaborating closely with Prof. Ludo Van Den Bosch's lab at VIB-KU Leuven, the team discovered that C21orf2 mutations lead to severe disruptions in the formation and integrity of primary cilia. Remarkably, they observed that nerve cells from ALS patients bearing these mutations exhibited a notable reduction in the number of cilia and experienced significant shortening of the cilia that did exist.
Implications of Cilia Damage
“It’s like having a kink in the cable,” explains Mathias De Decker, the study's leading author. 'When these cilia are impaired, crucial signaling pathways—specifically the sonic hedgehog (Shh) signaling pathway—become disrupted. This pathway is essential for maintaining the health of motor neurons. Without effective functioning of this pathway, neuronal connections to muscles at the neuromuscular junctions are jeopardized, leading to muscle dysfunction.'
Restoring Ciliary Function
In light of their findings, the researchers sought to explore whether these ciliary dysfunctions could be remedied. Further experiments demonstrated that by restoring levels of C21orf2 in damaged cells, they could normalize cilia structure and reinitiate the Shh signaling pathway, thereby reviving the connections between the nerve cells and muscles.
Broader Implications for ALS
Notably, similar functional impairments in cilia were also discovered in motor neurons from ALS patients with mutations in the C9orf72 gene—one of the most common genetic origins of ALS. This finding hints that cilia malfunctions are not constrained to a singular variant of ALS but may be a pervasive issue throughout the disease spectrum.
Future Directions
Prof. Philip Van Damme sees tremendous potential in these findings, emphasizing, 'While questions abound, our results open new doors to research. Repairing C21orf2 could address ciliary dysfunction and restore muscle connectivity. This indicates that targeting issues in cilia may represent a novel therapeutic strategy for ALS.'
Conclusion
This groundbreaking research ignites hope for those affected by ALS and underlines the urgent need for further exploration into cilia and their involvement in neurological health. As scientists dig deeper, we may be on the brink of unearthing revolutionary treatments that can alter the course of this devastating disease. Stay tuned for more updates, as the implications of this research could change everything for ALS patients and their families!




 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)