Revolutionary Discovery: 16 Genes Uncovered That Fuel Cancer Survival in Low-Oxygen Environments!
2024-11-05
Author: Rajesh
A groundbreaking study from the esteemed Johns Hopkins Kimmel Cancer Center has revealed a list of 16 genes that empower breast cancer cells to survive their perilous journey through low-oxygen environments. This critical discovery may hold the keys to preventing cancer recurrence and provides new therapeutic targets, one of which—MUC1—is already being tested in clinical trials.
Published in *Nature Communications*, this research shines a light on the challenging conditions within tumors, particularly hypoxia, where oxygen levels plummet due to rapid cell division. Lead researcher Dr. Daniele Gilkes explains how cancer cells that endure these conditions do not remain stagnant; they actively seek more oxygen-rich environments, often infiltrating the bloodstream, which can lead to metastasis—spreading cancer to distant body parts.
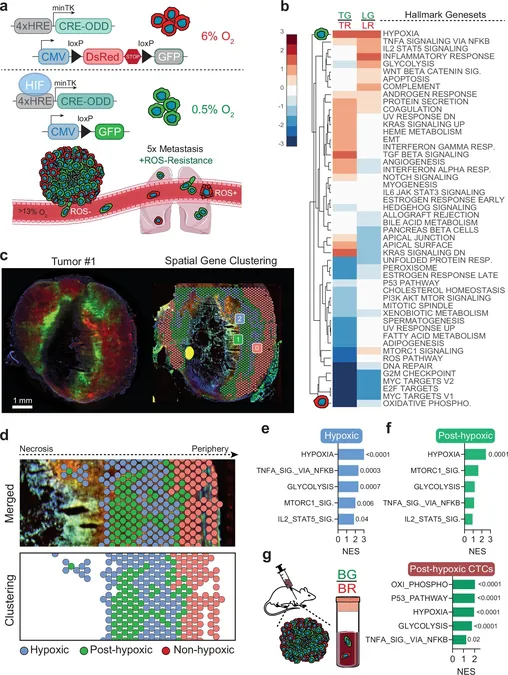
The researchers identified that these resilient cells leverage the protective functions of specific genes when facing reactive oxygen species, a type of stress encountered once they enter the bloodstream. The findings indicate that hypoxic cancer cells exist in close proximity to dead cells in a tumor and can migrate toward higher oxygen levels, seriously complicating treatment.
Further analysis showed that cells that survived extreme low-oxygen levels in tumors were significantly more adept at surviving in the bloodstream compared to less resilient cancer cells. The consequences of this are dire—after a tumor is removed, remaining cancer cells can metastasize, especially if the tumor was in a low-oxygen state, correlating with a worse prognosis for patients.
To investigate how these post-hypoxic cells manage to thrive in a hostile environment, Gilkes’ team utilized color-coding techniques alongside spatial transcriptomics. This approach revealed which genes remained activated as cells migrated from oxygen-deficient areas to more oxygen-rich regions. Surprisingly, certain hypoxia-induced genes maintained their expression well after leaving the tumor site.
The results hint at a “memory” effect where exposure to hypoxic conditions has lasting impacts on cancer cell behavior. This was particularly evident in laboratory models, where cells subjected to prolonged hypoxia exhibited characteristics similar to cells from actual tumors in animal models.
This research has profound implications, especially for patients suffering from triple-negative breast cancer (TNBC), notorious for its high recurrence rates. An analysis of TNBC biopsies revealed that patients whose cancer returned within three years had elevated levels of the MUC1 protein.
In their innovative experiments, Gilkes and her colleagues blocked MUC1 with a compound called GO-203, aiming to disrupt the survival mechanisms of these aggressive, post-hypoxic cancer cells. The findings were promising; minimizing MUC1 levels prevented these resilient cells from thriving in the bloodstream and significantly reduced metastatic formations in mice.
However, Dr. Gilkes cautions that there are multiple factors influencing cancer survival and metastasis, necessitating further research across various cancer types to validate these findings.
As of now, a phase I/II clinical trial is underway, targeting MUC1 to treat patients with advanced cancers, including those connected to breast, ovarian, and colorectal cancer. This work could potentially change the future landscape of cancer treatment—offering hope to many patients battling this formidable disease.
Stay tuned for more groundbreaking updates in cancer research!





 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)