Revolutionary Coating Technology Enhances Longevity of Implantable Biosensors, Paving the Way for Medical Innovations
2025-03-13
Author: John Tan
Introduction
In a groundbreaking development for healthcare technology, researchers from the Wyss Institute at Harvard University have unveiled a cutting-edge coating that significantly boosts the lifespan of implantable and wearable biosensors. These biosensors, crucial for monitoring biological molecules with minimal invasiveness, have vast potential in patient care, from diabetes management to assessing chronic diseases. Imagine having a diabetes monitor that not only lasts longer but provides more accurate real-time data—this could soon be a reality.
Current State of Wearable Devices
Currently, wearable devices like glucose monitors have become vital for individuals with diabetes, translating blood glucose levels into clear, continuous electrical signals. Beyond glucose, similar biosensors are being designed to track electrolytes in sweat and other biomarkers in bodily fluids, enabling more holistic health monitoring.
Challenges in Sensor Performance
However, the innovation comes with significant challenges. One of the primary barriers to longer-lasting sensor performance is a phenomenon called 'biofouling.' This occurs when bacteria, human cells, and other molecules accumulate on the sensor surface, severely limiting its function. These build-ups obstruct the sensors from accurately detecting their intended target molecules, compromising their ability to generate reliable electrical signals. Moreover, implanted biosensors can trigger inflammatory responses from the immune system, resulting in fibrotic tissue that can further impede their functionality.
Innovative Coating Solution
Addressing these issues could revolutionize the field, allowing for perhaps the first-time long-term monitoring of chronic or autoimmune diseases, enabling the assessment of new therapies in clinical trials, and facilitating crucial measurements in organs, including the brain.
Key Development by Wyss Institute
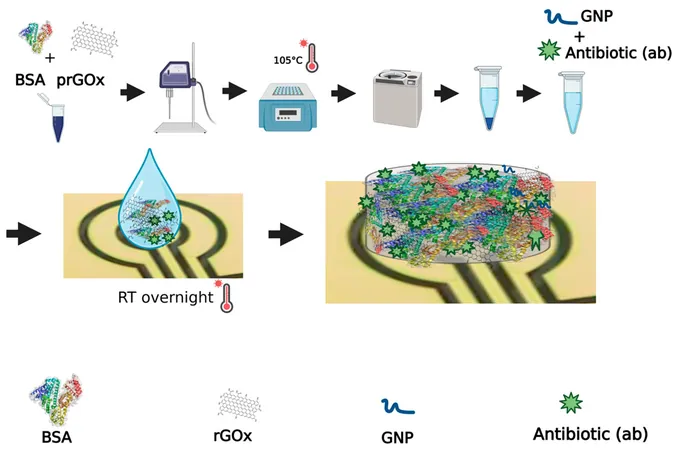
The revolutionary coating developed by the Wyss Institute's team presents a promising solution. The team has demonstrated that the application of this new coating on electrochemical sensor devices effectively inhibits the growth of Pseudomonas aeruginosa, a notorious bacterium responsible for antibiotic-resistant biofilms. This coating not only deters unwanted cell adhesion but maintains the sensors' detection capabilities for at least three weeks.
Expert Validation
Donald Ingber, M.D., Ph.D., the Founding Director of the Wyss Institute, highlighted the importance of this innovation, stating, 'With this novel coating technology, we have removed a central bottleneck in the development of next-generation electrochemical in vivo sensors. In the age of personalized medicine and digital health, it opens up a multitude of diagnostic and research applications.'
Technical Aspects of the Coating
The coating, which features a cross-linked lattice of bovine serum albumin (BSA) and functionalized graphene, ensures superior electrical signaling, while forming a protective barrier against various contaminants. This advancement allows for the stable inclusion of antibodies essential for detecting specific analytes, along with antibiotic agents to combat biofouling effectively.
Practical Testing Outcomes
In practical tests, the team successfully tracked two critical inflammation biomarkers over more than three weeks using sensors exposed to complex human plasma. During this period, the new coating thwarted the attachment of fibroblasts and the formation of biofilms linked to Pseudomonas aeruginosa, while remaining undetected by pro-inflammatory immune cells.
Production and Future Applications
Significantly, this protective coating can be produced from affordable materials and through a straightforward scalable process. This allows for the mass production of biosensors that can have immediate consequences for patient care and scientific advancement. The Wyss Institute has already patented this innovative technology and is actively seeking partners to transition it into real-world applications.
Conclusion
As the healthcare industry moves toward a future personalized by technology and precision medicine, the potential of this new biosensor coating could change the landscape, offering enhanced monitoring capabilities and improved patient outcomes across various medical fields. This remarkable development is a testament to the possibilities that emerge from interdisciplinary collaboration and innovative thinking in biomedical engineering.

 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 Česko (CS)
Česko (CS)
 대한민국 (KO)
대한민국 (KO)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)
 الإمارات العربية المتحدة (AR)
الإمارات العربية المتحدة (AR)