Revolutionary ChemoID Test Offers Hope for Platinum-Resistant Ovarian Cancer Patients
2025-04-04
Author: John Tan
Introduction
In a groundbreaking Phase 3 trial recently published in the journal npj Precision Oncology, researchers revealed that a novel cancer stem cell test, known as the ChemoID platform, can significantly improve treatment outcomes for patients suffering from platinum-resistant ovarian cancer. This exciting advancement could reshape how treatment decisions are made, promising a brighter future for those battling this challenging condition.
Understanding Platinum-Resistant Ovarian Cancer
Dr. Thomas Herzog, the chief investigator from the University of Cincinnati Cancer Center, emphasized that while epithelial ovarian cancer may initially respond well to chemotherapy, many patients eventually experience a relapse as the tumors develop resistance. This resistance is largely attributed to the activation of cancer stem cells (CSCs), which have the remarkable ability to rebuild tumors after chemotherapy has disrupted them.
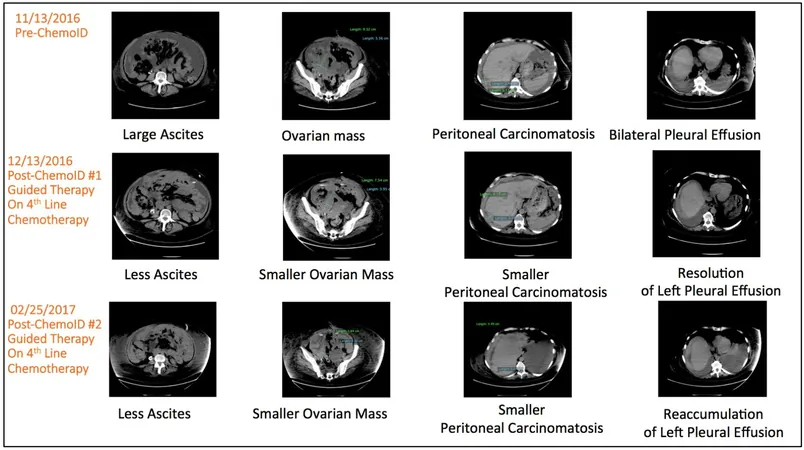
The ChemoID Platform
The ChemoID platform takes a unique approach by testing individual patients' CSCs against various anticancer drugs to pinpoint the most effective treatment options. Dr. Pier Paolo Claudio, who co-developed this innovative clinical test, stated, “By assessing not only the tumor cells but also the cancer stem cells for chemosensitivity, we can target and eliminate the very cells that could lead to a tumor's resurgence.”
The Clinical Trial
In this pivotal study, 81 patients with platinum-resistant ovarian cancer—defined as cancer recurrence within six months following platinum-based chemotherapy—were randomly assigned to receive treatment either based on ChemoID recommendations or according to standard physician judgment. Typically, physicians consider approved therapies, past treatment responses, and potential side effects when determining a treatment plan.
Trial Outcomes
The trial’s primary evaluation metric was the objective response rate (ORR), which gauges the proportion of patients achieving a partial or complete response to treatment. Secondary endpoints included progression-free survival (PFS), the duration that patients can live without their cancer worsening, and the duration of response to treatment.
Remarkably, results showed a significant boost for patients treated via the ChemoID platform: the ORR was an impressive 50%, in stark contrast to just 5% for the standard physician-choice group. Furthermore, the median PFS for ChemoID patients soared to 11 months versus a mere three months for those whose treatments were not guided by the test. Additionally, patients in the ChemoID arm enjoyed a median duration of response of eight months, compared to five-and-a-half months for their counterparts.
Implications for Healthcare
These promising results indicate that more effective treatments could not only enhance patient outcomes but also lead to substantial savings in healthcare costs related to the management of ineffective therapies and their associated toxicities. Dr. Claudio noted that this study’s findings could alleviate the financial burden often faced by ovarian cancer patients, particularly those grappling with treatment-resistant forms of the disease.
Future Directions
Looking ahead, Dr. Herzog stated that further research is essential to validate the ChemoID platform and to explore its effectiveness across various molecular subgroups, including patients with specific BRCA mutations. Additionally, the potential incorporation of new biologic therapies into treatment regimens may help refine the applications of this innovative test.
Conclusion
This remarkable development marks a significant step toward personalized treatment strategies for ovarian cancer, holding the promise of not just improved responses but also greater hope for patients grappling with this challenging illness. Is this the breakthrough we've been waiting for in cancer treatment? Only time will tell as further research unfolds.


 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 Česko (CS)
Česko (CS)
 대한민국 (KO)
대한민국 (KO)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)
 الإمارات العربية المتحدة (AR)
الإمارات العربية المتحدة (AR)