
Revolutionary Catalyst Breakthrough Could Transform Microplastics Into Sustainable Fuel
2024-11-07
Author: Rajesh
Microplastics, the tiny plastic particles less than 5 millimeters in size, are wreaking havoc on our planet. These pervasive pollutants contribute to global warming, disrupt ecosystems, and contaminate our food chains with harmful chemicals. This alarming reality has prompted scientists like Dr. Manish Shetty to take action, striving to eliminate plastics before they infiltrate the environment.
Dr. Shetty's groundbreaking research focuses on the development of sustainable chemicals and advanced waste management strategies that could pave the way for a cleaner future. Central to this study is the innovative use of catalysts that aim to make green hydrogen accessible for effective waste management.
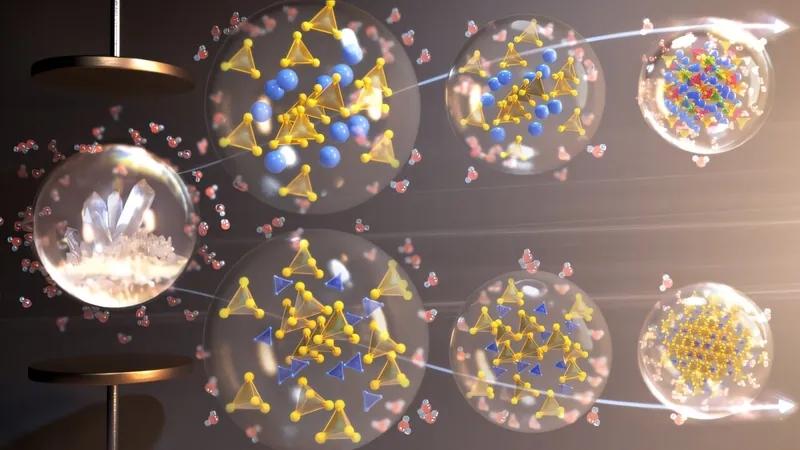
Utilizing minimal amounts of solvents, which also serve as hydrogen sources, Dr. Shetty's team targets a specific type of plastic known as condensation polymers. These include commonly used materials like polyethylene terephthalate (PET)—often found in bottles, packaging, and textiles.
"What we have achieved in this study is the ability to decompose condensation polymers into aromatic compounds that can be repurposed as fuels," Shetty explained. "By employing liquid organic hydrogen carriers, we can store hydrogen and deploy it to break down these polymers effectively."
The team has ingeniously designed catalysts that capitalize on the hydrogen released from these organic carriers once the condensation polymers are broken down. This innovative approach is detailed in Dr. Shetty's recent publication in *Angewandte Chemie International Edition*.
Their findings reveal that these catalyst surfaces can effectively transform PET into p-xylene, a versatile molecule that can serve not only as a fuel but also as a building block for valuable chemicals. The implications of this research extend beyond waste management; it presents a sustainable solution critical for the future of the chemical industry.
"We are not just addressing waste management issues—our research offers a pathway to sustainability through these catalysts,” Shetty emphasized. “These organic molecules act as transporters of hydrogen, taking it from its point of generation to its application in urban waste management, where a vast amount of plastic waste accumulates."
To further break down PET into useful fragments, the research indicates that methanol will play a dual role as both a reducing agent and a source of hydrogen to convert PET into p-xylene.
Dr. Shetty is optimistic that the applications of this research could revolutionize our economy by reducing its dependence on fossil fuels.
"As green hydrogen becomes more available—especially through methods like water electrolysis—we will desperately need effective hydrogen carriers as transport vectors," he remarked. "One promising application is within waste management and the valorization of discarded materials."
With the potential to transform the way we handle plastic waste and fuel, Dr. Shetty's research may just be the key to a more sustainable and eco-friendly future. Could this breakthrough finally provide the solution we need to combat the microplastics crisis? Only time will tell.




 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)