Revolutionary Carbon Nanohoop Unlocks Controlled Iron Release with Light!
2025-03-26
Author: Nur
Introduction
In an exciting breakthrough, researchers from the Universities of Amsterdam and Zurich have unveiled a cutting-edge molecular system that enables the controlled release of iron. This innovative approach integrates a compound known as ferrocene, a molecular structure that encases an iron atom, with a sophisticated carbon "nanohoop."
Publication and Research Team
Published in the prestigious Journal of the American Chemical Society (JACS), this study marks a significant advance in the use of photocaging technology—a method that utilizes light to precisely manipulate molecular activity. Led by Dr. Tomáš Šolomek at the University of Amsterdam and Dr. Peter Štacko at the University of Zurich, the researchers aim to harness the power of light for biomedically relevant applications.
Significance of Photocaging Technology
Photocages have gained recognition for their ability to activate important biological molecules, such as proteins and drugs, offering a deeper understanding of biochemical mechanisms. Moreover, they present potential therapeutic applications, including cutting-edge photoactivated chemotherapy, that could revolutionize cancer treatment.
Iron in Human Biology
The study shifts focus to iron, an essential element in human biology known for its crucial role in processes like oxygen transport, cellular energy production in mitochondria, and safeguarding the body against oxidative stress. Unlike complex natural systems that manage iron uptake and balance, the researchers propose a simplified synthetic equivalent capable of storing and releasing iron on demand.
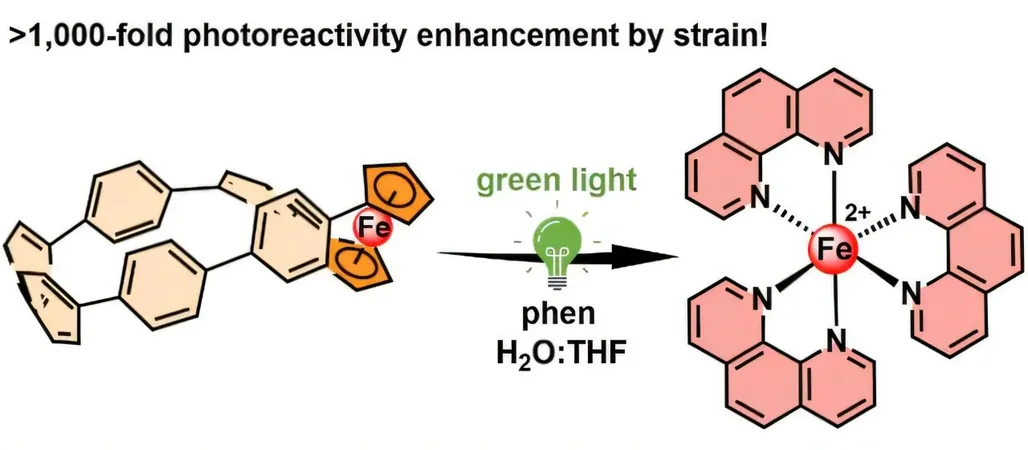
The Core System: Ferrocene and Nanohoop
At the heart of this system lies ferrocene, a stable organometallic compound that typically exhibits remarkable chemical resilience. But when incorporated into a carbon nanohoop constructed of interconnected benzene rings, the stability is altered. This design creates significant mechanical stress within the nanohoop structure, making it sensitive to green light irradiation. Upon activation, this light triggers the release of iron ions with impressive efficiency.
Implications and Future Directions
The implications of this research are vast. Beyond merely enhancing iron release, the concept of utilizing mechanical stress within molecular systems paves the way for the development of new responsive materials across various fields, including supramolecular chemistry, organometallic chemistry, and polymer chemistry. This revelation could lead to innovative technologies tailored for dynamic responses to environmental stimuli, pushing the boundaries of material science and biotechnology.
Conclusion
Stay tuned as this exciting work progresses; the future of pharmacology and materials science may very well depend on controlled molecular systems like this revolutionary carbon nanohoop!




 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 Česko (CS)
Česko (CS)
 대한민국 (KO)
대한민국 (KO)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)
 الإمارات العربية المتحدة (AR)
الإمارات العربية المتحدة (AR)