Revolutionary Carbon Catalyst Harnesses Airborne Oxygen for Efficient Green Hydrogen Peroxide Production!
2025-03-17
Author: Mei
Introduction
In a groundbreaking development, researchers have unveiled a game-changing method for producing hydrogen peroxide, one of the globe's top 100 industrial chemicals, widely utilized in sectors such as chemicals, healthcare, and semiconductors. Traditionally, hydrogen peroxide has been manufactured through the anthraquinone process—an approach marred by significant drawbacks, including exorbitant energy consumption, reliance on costly palladium catalysts, and environmental pollution stemming from hazardous by-products.
The Need for Greener Alternatives
However, a new wave of research is shifting toward greener alternatives, particularly the electrochemical reduction of oxygen using budget-friendly carbon-based catalysts. Although promising, this approach has faced hurdles due to the high cost of injecting high-purity oxygen and the instability of the generated hydrogen peroxide in basic electrolyte environments.
Innovative Solutions from KIST
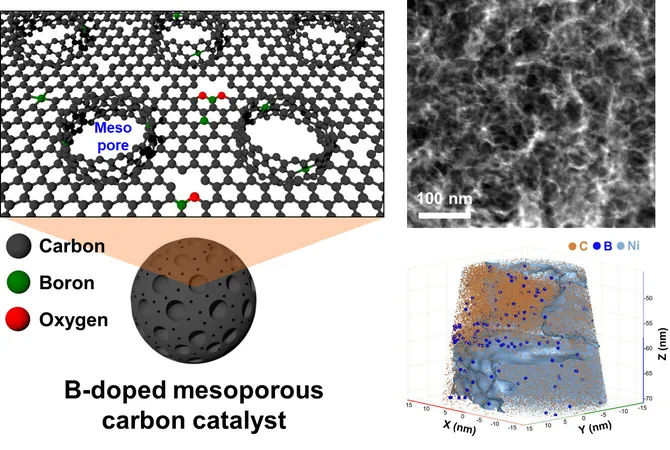
To tackle these challenges, a pioneering team led by Dr. Jong Min Kim from the Center for Extreme Materials Research at the Korea Institute of Science and Technology (KIST) has developed a highly efficient mesoporous catalyst. This innovative catalyst can generate hydrogen peroxide even in air conditions with low oxygen concentrations and neutral electrolytes. By integrating mesopores into the carbon catalyst structure, the team has significantly enhanced its functionality.
Research Findings
Their research, published in the prestigious journal Advanced Materials, details the synthesis of boron-doped carbon featuring mesopores approximately 20 nanometers wide. This was achieved through a reaction involving the greenhouse gas carbon dioxide (CO2), the highly effective reducing agent sodium borohydride (NaBH4), and meso-sized calcium carbonate (CaCO3) particles. The final product emerged after selectively eliminating the calcium carbonate, resulting in a sophisticated catalyst designed for optimal performance.
Enhanced Catalytic Activity
The experiments validate that the unique curved surfaces created by the mesopores amplify the catalytic activity even in neutral electrolyte environments—settings where hydrogen peroxide production typically struggles. Real-time Raman analysis has confirmed that this mesoporous architecture effortlessly facilitates the transfer of oxygen, helping maintain impressive catalytic efficiency in environments with an oxygen concentration as low as 20%.
Significant Implications
The implications of these findings are monumental. The team demonstrated that their boron-doped mesoporous carbon catalysts could achieve hydrogen peroxide production efficiencies surpassing 80% under conditions that closely resemble commercial production—neutral electrolytes, air supply, and a substantial industrial-scale current density of 200 mA/cm². Remarkably, they synthesized hydrogen peroxide solutions boasting a concentration of 3.6%, exceeding the standard medical grade of 3%, which suggests promising prospects for commercialization.
Expert Commentary
Dr. Jong Min Kim stated, "Our mesoporous carbon catalyst technology harnesses oxygen from the air we breathe to generate hydrogen peroxide in neutral electrolytes. This method is not only more practical than traditional catalysts but will also accelerate the industrialization of hydrogen peroxide production."
Conclusion
As the world moves towards greener manufacturing processes, this revolutionary approach could reshape the landscape of hydrogen peroxide production, making it more sustainable, efficient, and economically viable. Stay tuned for more updates on this incredible innovation that holds the potential to transform multiple industries!



 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 Česko (CS)
Česko (CS)
 대한민국 (KO)
대한민국 (KO)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)
 الإمارات العربية المتحدة (AR)
الإمارات العربية المتحدة (AR)