Revolutionary Cancer Vaccine Emerges Amid Groundbreaking Research
2025-09-04
Author: Mei
A New Era in Cancer Treatment
In a significant breakthrough for cancer therapy, researchers have shed light on the complexities of immune-checkpoint therapy (ICT)—a treatment that harnesses the body's immune system to combat cancer. Despite its impressive advancements over the past two decades, many patients still see limited results, particularly due to immune-evasive (or ‘cold’) tumors. This shortfall has sparked urgent research into why some patients do not respond to ICT.
Dr. Scott Lippman, a leading medical professor at UC San Diego, emphasizes the necessity of pinpointing non-responders to optimize precision therapy and avoid the financial burden of ineffective treatments. His latest research, featured as a cover story in the Journal of Thoracic Oncology, offers groundbreaking insights that not only address these issues but have also led to the development of a novel cancer vaccine poised to enhance ICT's effectiveness.
The Genetic Breakthroughs Behind Cancer Resistance
Lippman’s previous investigations laid the groundwork for this new study. His team uncovered that specific genetic alterations in head and neck cancers, particularly related to the human papillomavirus (HPV), are a primary factor in nonresponse to ICT. They discovered that a loss of genetic material on chromosome 9p is significantly correlated with immune evasion.
This finding catalyzed a flurry of research confirming that about 15-20% of tumors exhibit this 9p chromosomal loss, a phenomenon not just found in head and neck cancers but also in lung, mesothelioma, melanoma, and bladder cancers. This genetic insight has led to the development of clinical tests aimed at predicting ICT effectiveness.
Unraveling the Molecular Mysteries
While understanding the genetic landscape was crucial, Lippman’s team sought to uncover the molecular details connecting 9p loss to ICT resistance. In a stunning revelation, they identified deficiencies in type-I interferon genes as a critical mechanism driving immune evasion, overshadowing previously known factors. This pivotal finding underscores how tumor-specific genetic alterations can inhibit immune cell infiltration and the production of crucial signaling proteins.
Dr. Teresa Davoli, a co-senior author of the study, noted that the absence of type-I interferons in tumors creates an immunity-favorable environment, hindering the immune system's ability to combat cancer effectively.
A Game-Changing Cancer Vaccine
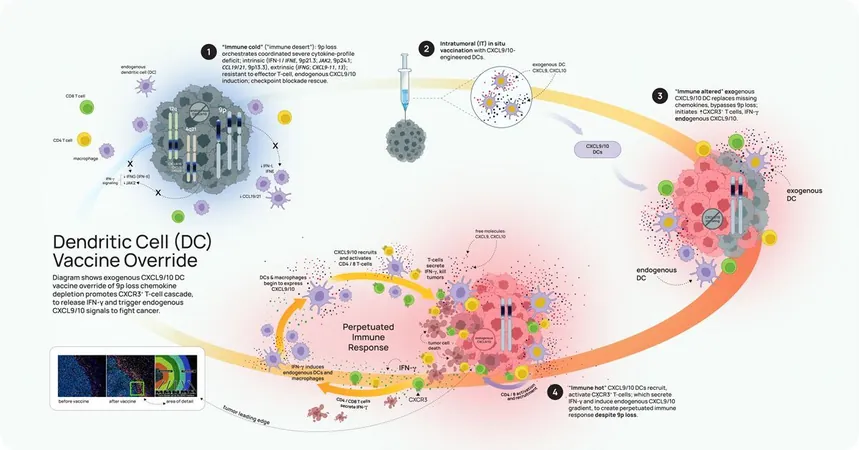
Building on these insights, Lippman and his colleagues initiated a comprehensive study encompassing multiple genomic datasets and engineered mouse models, leading to the innovation of a new immune-cell vaccine. This vaccine aims to target the unique challenges posed by tumors characterized by 9p-loss.
The vaccine uses engineered immune cells, known as dendritic cells, to bypass the depletion of critical chemokines like CXCL9 and CXCL10, ultimately triggering a more potent anti-tumor response.
Although initial trials have shown promise in animal models, researchers acknowledge that further investigation is necessary to gauge the vaccine's potential in clinical settings. As Dr. Steve Dubinett of UCLA stated, understanding the intricacies of immune responses at a cellular level marks a significant step forward in personalized cancer therapy.
Hope on the Horizon
The implications of these findings could reshape how cancer treatment is approached, specifically in tailoring vaccines that are more specific than traditional ICT methods. As researchers continue to explore the intricate connections between genetic changes and immune responses, the future looks bright for patients hoping for effective therapies. According to Lippman, this breakthrough represents a monumental leap forward in the realm of personalized cancer treatment that could change countless lives.





 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 Česko (CS)
Česko (CS)
 대한민국 (KO)
대한민국 (KO)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)
 الإمارات العربية المتحدة (AR)
الإمارات العربية المتحدة (AR)